Achieving Regulatory Success in Phases IIIb and IV
Well-planned studies designed to pinpoint additional data are increasingly important as a result of the EU's emphasis on added therapeutic value.
The explosive costs associated with today's clinical trials require a very high level of scrutiny to weigh their scientific and regulatory merits prior to their commencement. However, passing the regulatory hurdles in order to obtain a marketing license does not always fully coincide with providing the necessary clinical information to place a new product in its intended context. Phase IIIa studies are therefore often not sufficient from a public health viewpoint, and additional Phase IIIb and IV trials need to be considered. This article gives a review of some of the regulatory challenges associated with later-phase development and highlights opportunities to participate in the formation of guidelines.
Definitions
While various definitions of what Phase IIIa, IIIb, and IV constitute are in use between various agencies, academic centers, and companies, the following definitions are used in this article for the purpose of clarity:
Phase III: The efficacy and safety trials that are conducted with the primary purpose of filing for a marketing authorization (MA) of a particular product with the relevant authorities.
Phase IIIa: The efficacy and safety trials that support the initial claims made by the future marketing authorization holder.
Phase IIIb: Additional studies that are conducted to increase patient exposure; these additional data are either provided to the authorities at the time of approval or, because the studies are typically not completed at the time of initial filing, will be submitted as a postapproval commitment.
Phase IV: Studies with focus on new indications, new comparators, new efficacy endpoints, efficacy assessed by new methods, etc. after MA has been obtained.
These definitions are not universally recognized. Most regulatory agencies do not make a distinction between Phase IIIa and IIIb, and some companies use the concept of a Phase V that would fit under the definition of Phase IV above. There is also considerable overlap. Some studies are conducted to provide information that can be used to promote a drug at the time that a license is granted, such as a study in a special population or under special circumstances. When studies are initiated before the filing for a license, they are to be considered Phase IIIb studies, but there is often clear similarity in objectives or design to Phase IV studies.
The results of a Phase IIIb study are, as previously stated, typically not available at the time of initial filing for a license. The results must be presented to the regulatory authorities when available. Furthermore, the authorities must be informed of the status and kind of all additional ongoing incomplete studies at the time of application submission.
Challenges in drug development
It is estimated that out of every 10,000 molecules discovered, synthesized, and screened, only about 50 are found to have potential as a drug and progress to preclinical in vitro and animal testing. Further, only 10 make it to Phase I and just three make it to Phase II, leading hopefully to one product that can get licensed as a human medicine. As Figure 1 illustrates, the basic research takes on average 3 to 7 years, the clinical development an additional 4 to 8 years, and registration another 1 to 2 years for an accumulated average of 10 to 14 years on a product that is granted a 20-year patent.
1
This long lag time between the development of a concept to roll-out of a new product is exceptionally long compared to other industries; in the car industry this is typically in the range of 2 years, in the software industry less than 18 months. This lag time adds to the investment burden.
According to a May 2003 report from the Tufts Center for the Study of Drug Development,2 the investment to develop one new product is now on average almost $900 million (E 730 million or £500 million), with clinical development expenses increasing in particular. It is thus fair to say that the development of new drugs for humans is a high-risk process that requires a very high investment over a long period of time, with severe attrition rates of initially screened compounds that may lead to very few licensed products. This explains why there is a tendency to bring these new products to the market as soon as safely possible despite a progressed stage of development, as the anticipation of the public and the industry is great.
In the current high-risk environment, priority is given to develop drugs in possibly contradictory categories: drugs that are safe, that belong to franchises that correspond to the highest public health needs, with few or no competitors, and with achievable targets to fulfill all regulatory requirements. Unfortunately, many of these factors oppose further development and can lead to program shutdown when the risk is deemed too high.
There are ongoing and considered initiatives underway to tip the balance more favorably back towards development, such as accelerated Phase II and III trials, shortened review time to obtain licenses, and added data protection times. As Figure 2 shows, it still takes an average of 1.5 years to obtain a marketing approval in most major markets despite many sincere initiatives to shorten approval times. Figure 3 shows detailed registration times for Centralized Procedures with the European Agency for the Evaluation of Medicinal Products (EMEA), confirming a 1.5-year average, with nearly half of the time used for assessment by the agency, an equal amount to respond to questions by companies, and limited time for the decision-making process and administration. As a result, major changes are unlikely given the complexity of drug approval. It has been well discussed that while detection of new chemical entities is fairly steady when seen over a longer period, the costs have been rising over the last decade while the actual number of products has been falling. This trend is unlikely to reverse.
From both public health and marketing points of view, it is very important to get new therapies to patients as soon as safely possible. An important aspect in late-phase development is how to obtain early MA for the product. This is the focus of Phase IIIa studies, often based on science and regulatory requirements that differ from country to country.
The choice of a comparator drug is a good example of the challenges. The current medical practice drug, the gold standard therapy drug, and the market leader drug often are not synonymous, especially in different regions. While regulators may request a comparison to the current or gold standard, the comparison to all three can be important, especially from patient and health care provider perspectives because that will eventually be the choice they will have to make.
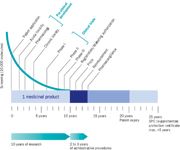
Figure 1. The pharmaceutical R&D process. Courtesy of the European Federation of Pharmaceutical R&D Process.
Drivers of Phase IIIb and IV
There are two reasons to consider Phase IIIb studies: they are done either at the request of agencies, or are driven by the needs of the industry. When a regulatory agency deems that a product can be brought to market even though some information is mis-sing from the paperwork, a conditional approval is given along with a request for additional data. It is good to remember that additional information can be requested by the agency with any new license. That request can be a follow-up measure communicated at the time of approval, and can include data from the preclinical or clinical area, pharmacovigilance data, better outcomes data, etc.
Yet an "approval under exceptional circumstances" can be granted in cases where there is an unmet medical need in a well-defined population, and can be granted with less than fully comprehensive datasets. The approval process typically is accelerated, but still leads to specific obligations that the Market Authorization holder needs to fulfill in order to keep the license. Both specific obligations and follow-up measures are postapproval commitments that are usually conducted as Phase IIIb trials. A restricted license usually becomes unrestricted upon fulfillment of these commitments.
Companies too may define a need for data above what is needed to obtain a license, and this constitutes the second set of drivers for Phase IIIb studies. Common drivers include a better understanding of the benefit/risk balance, data in specific subpopulations (e.g., children and the elderly), identification of less common adverse reactions, refined dosing recommendations, comparison of the new product to the current medical/gold/ market-leader standard, or trials for indications for which use of the drug is presumed once available.
Other strategic considerations to obtain more data may include a need for support in price and reimbursement applications after the license is approved, establishment of the added value of the new therapy in a particular marketplace, adjustment to existing practice guidelines, or a request to change aspects of the formal indications such as the therapeutic indications (section 4.1) or pharmacodynamic properties (section 5.1). The concept of added therapeutic value is particularly important in the European Union (EU), even though a recent proposal to include it as a requirement for regulatory approval in the new EU legislation (the list currently includes quality, safety, and efficacy) was not adopted by the European Parliament. Added therapeutic value, often discussed by the EMEA and most national agencies, has an impact on guidelines and can influence general use and reimbursement.
Planning for Phase IIIb studies is mainly done at very early stages of development. Since these studies typically start but are seldom completed in time for approval, it is important that agencies are in agreement with a cutoff time for data to be submitted with the original filing. That time may need to be negotiated: Phase IIIb studies cannot start before deadline is established.
Regulatory approval and various guidelines
It is easier to obtain an MA when there are clear, undisputed clinical practice guidelines that can be fully adhered to during development of the product. This is rarely the case, however, as most guidelines evolve with new acquired medical knowledge. Guidelines for drug development are developed by agencies and are often based on existing guidelines put forward by medical societies, government agencies, and specialist societies.

Figure 2. Median approval times in the major markets, 1997-2001. Courtesy of CMR International.
A part of this new knowledge is derived from clinical trials, particularly the large-scale studies conducted in Phase III and IV. For a new drug, the end label obtained at time of approval can itself further influence the existing clinical treatment guidelines. A new gold standard can now get established in cases where there was no existing therapy. The treatment guidelines, which can have a large impact on the future utilization of the product, may change.
Most countries in the EU have organizations that set up guidelines. They have a great variability in their impact, both directly (in case of mandatory binding practice guidelines), or indirectly (nonreimbursement of therapy outside the recommended guidance, which has a very large impact in the EU). There are selected opportunities for the industry to contribute to this process. A few examples follow below.
United Kingdom: In 1999 the National Institute for Clinical Excellence (NICE) was established in England and Wales. The primary objectives of NICE are to provide guidance through appraisals of health technologies, both pharmaceuticals and other health interventions, and to publish clinical guidelines for management of certain conditions. Currently, NICE has completed over 75 appraisals and has 40 more in progress. These appraisals, of course, have either a negative or a positive impact on the potential of a new medicinal product, depending on how it is viewed.
NICE has become arguably the most influential national clinical guideline body on an EU level. In the appraisal process, NICE solicits and encourages direct input from a variety of stakeholders, such as patient organizations, health care professional organizations, and industry with an interest in the assessed technology. This stakeholder process allows for industry to influence the contents of the guidelines through open communication with the organization. This process is transparent, since application forms are posted on its Web site.3 NICE is a part of the National Health Service (NHS), and has a mandate to provide current, robust, and reliable guidance to patients and health care professionals. Phase III and IV studies for pharmaceuticals are the main drivers for the assessment of "a technology" (meaning a product), and this appraisal is an important factor in ensuring a market takes up a new medicinal product.
Germany: Germany, too, has many organizations developing guidelines. The more visible ones are the Bundesaerztekammer (German Medical Association) and the Kassenaerztliche Bundesvereinigung (Federal Association of Sick Funds' Physicians). The Aerztliche Zentralstelle fuer Qualitaetsicherung (Physicians' Agency for Quality in Medicine) has a department to evaluate the relevance of new guidelines. The Arzneimittelkommission der Deutschen Aezteschaft (Drug Commission of German Physicians) also issues guidances, but these do not have economic references as NICE does. There are significant changes, but traditionally the organization with the most impact has been the Bundesausschuss der Aerzte und Krankenkassen (Federal Committee of Physicians and Sick Funds), which issues binding guidelines to which physicians have to adhere and are to some degree monitored. This organization consults with industry associations but not with individual companies.
France: Most French guidelines are developed by a French regulatory agency (AFSSAPS) and the Agence Nationale d’Accréditation et d’Evaluation en Santé (ANAES) The different types of guidelines include good practice guidelines, guidelines for clinical practice, medical references, and consensus conferences. An important player in France is the Transparency Commission, which puts a new drug treatment into perspective among the current treatment options. The evaluation, performed when the company requests reimbursement for a product, is called a transparency notice, which then serves as the basis for the reimbursement of the product. Therefore, data of even Phase IIIa trials can have a large impact on the reimbursability of the product, which becomes a major determinant in the product's use. AFSSAPS also publishes "transparency sheets" that include the "opposable medical references," an original approach accessible by the general public which informs physicians what not to do.
Sweden: Sweden's Medical Products Agency (MPA), together with the Norwegian regulatory agency, issues treatment recommendations for disease areas. There is a Technology Assessment Board (SBU) similar in its structure to NICE, which also issues guidelines on specific technologies. None of these guidelines are binding, but they influence prescribing patterns in the country. Furthermore, local and regional formularies develop lists of recommended drugs for physicians to prescribe for particular conditions. In most regions, these recommendations are not binding. However, in some areas, such as Stockholm, physicians extensively use a computer-based prescription system interconnected with the formulary software, leading to a reminder at the time of entering the script. In these regions, the recommendations are followed to a much larger degree.
Phase IIIb and IV studies: The negatives
While many positive aspects of Phase IIIb and IV studies are highlighted above, there are negative considerations as well. Price is a major obstructive factor: large Phase III and IV studies can be very costly, with no guarantees of success. Approval is sometimes not obtained or is delayed, product characteristic changes are not achieved, products are classified as not reimbursed, and so on. The less-than-ideal transparency of procedures within most agencies and the lack of clear foresight of the outcomes are strong negative factors. Further, new indications can lead to new follow-up measures and more studies.
These activities cause an increased workload for various departments in the industry, not least regulatory affairs. The extra workload on a regulatory affairs group needs to be carefully balanced against other ongoing critical programs and activities, or these will receive less attention.
Conclusions
Phase IIIb and IV studies can be done at request of regulatory agencies and can sometimes help get new products better established in a particular context. The design of Phase IIIb and IV studies are often the result of existing guidelines, but the results of the studies can in turn impact these guidelines. In the EU, the concept of added therapeutic value is very important. The investment in cost and manpower is considerable, and the risk of nonapproval is always real. All these positive and negative factors need to be carefully brought in balance at an early stage.
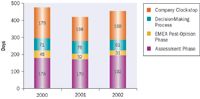
Figure 3. Average approval times for products in the EU centralized procedure, 2000-2002 (derived from EMEA Annual Report 2002).
References
1. EFPIA. The Pharmaceutical Industry in Figures. Key Data 2003 Update. http://
www.efpia.org/6_publ/Infigures2003.pdf
2. Tufts Center for the Study of Drug Development. A methodology for counting costs for Pharmaceutical R&D. http://csdd.tufts.edu/NewsEvents/RecentNews.asp?newsid=29
3. http://www.nice.org.uk
The material in this article was presented at the Applied Clinical Trials Winter Forum meeting in London, U.K., on 21 November 2003.
Alain Bouckenooghe, MD, MPH, is an independent consultant, +32 16 445 063, email: vlampi@hotmail.com.

What Can ClinOps Learn from Pre-Clinical?
August 10th 2021Dr. Hanne Bak, Senior Vice President of Preclinical Manufacturing and Process Development at Regeneron speaks about her role at the company as well as their work with monoclonal antibodies, the regulatory side of manufacturing, and more.