Single IRB Review for All Multicenter Clinical Trials
Since the emergence of the independent IRB sector, sponsors have found that regulatory and ethical review by a single IRB yields important benefits with respect to efficiency, high quality, and consistency in human research protections.
When will the industry’s sponsors of multicenter clinical trials mandate single IRB review1 once and for all?
Since the emergence of the independent IRB sector, sponsors have found that regulatory and ethical review by a single IRB yields important benefits with respect to efficiency, high quality, and consistency in human research protections (Figure 1).
Figure 1. A depiction contrasting multiple re-reviews of a single protocol by many local IRBs with single review by one IRB. Note that with single review, a decision to approve the research is typically achieved in a approximately one-week. In contrast, multiple redundant reviews often occur over the course of many months, an inefficient process. The buildings represent institutions and their review boards, and the multiple colors and types of the local IRBs depict the wide range of variability in expertise, process, and quality of reviews.

Nevertheless, today the biopharma and medical device industries tolerate re-review of clinical trials by multiple local (sometimes non-AAHRPP accredited2) IRBs for most clinical trials. This tolerance wastes time and money and lengthens the development timelines for investigational products.
That timelines for review are shorter for a single review vs. multiple IRB re-reviews of research is not only intuitively logical, it also is supported by facts. As shown in Figure 2, the time from submission to approval (Turnaround Time) at local IRBs requires an average of 46 days for a convened review.3 These data are derived from 131 different institutions, some of which are accredited by AAHRPP, and some not. Similar observations (40-day Turnaround Time) were reported from a cohort of AAHRPP-accredited-only institutions.4 By contrast, the Turnaround Time for convened, single IRB review for typical multicenter clinical trials is much shorter, typically 3 to 8 days.3.5
Figure 2. Average Turnaround Time (Days) for convened IRB Local Re-review vs. Single IRB Review.
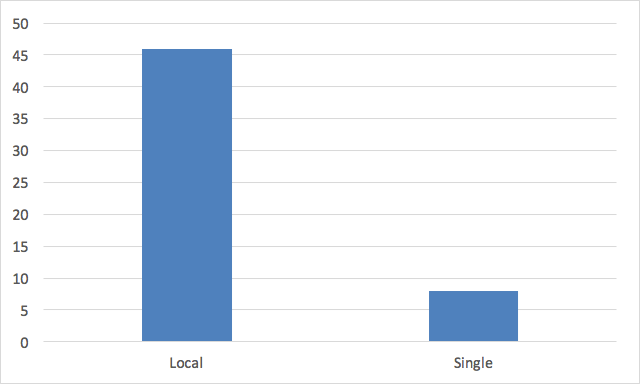
Sources for figure 2. can be found here.
The greater time benefit of single review of multicenter clinical trials is derived from the fact that all, but the first site is added via the expedited procedure, a process that requires about 1 to 3 days, depending on the institution’s unique requirements.3 For a sponsor or CRO conducting multicenter clinical trials, the differences are much larger. Typically, each institution submits on its own timeline and each re-review requires a convened IRB and cannot occur via expedited review. This multiplies the difference manifold, resulting in months passing before all sites have local IRB approval.
The reasons sponsors accommodate this practice are multi-faceted, but frequently stem from sponsors’ desire to maintain strong positive relationships with all sites, including those with local IRBs. Sponsors may also be resistant to being directive about sites’ internal practices.
On occasion, industry does make exceptions to the tolerance of local re-review. For example, single IRB review is required for the Novartis Signature Series of oncology trials6, the Quintiles Precision Enrollment program7, and other cases when the enrollment period is short and only the fastest starting sites are selected. Yet on the whole, sponsors encourage but do not require central review.
Some of the most frequently used sites retain their position that only the local IRB can best protect the rights and welfare of their patients who volunteer to participate in clinical trials. Yet time has shown that central review of multicenter research has important advantages over re-review at local IRBs and patients are frequently cited as stating that minimizing the time and administrative burden to enrolling in clinical trials is very important to them.
For example, in addition to the quality, consistency and efficiencies that single IRB review provides, a sponsor or CRO’s administrative burdens are a fraction of what is required compared with dealing with multiple IRBs, with different requirements at each site. Managing multitudes of IRB approval documents for a single study requires complex processes and dedicated resources, lengthening the time before research can commence, without adding to human research protections and increasing opportunities for administrative error that might have regulatory consequences.
Importantly, the industry’s emerging focus on patient-centricity has driven progress towards emerging technologies, such as eConsent and wearable devices. These technological and patient-focused enhancements are best reviewed by central IRBs because multiple adaptations to meet the requests of local IRBs may not be possible, or can only be made with significant negative impact on time, yielding attendant financial consequences.
Institutions benefit from single IRB review by accelerating study startup in the absence of local IRB review, and thus have an opportunity to enroll more patients within competitive enrollment timelines. In addition, single-site, non-industry-sponsored research-which is generally not as well-vetted as multicenter clinical trials funded by industry-can receive the focus it deserves by local IRBs that are relieved of the burden of reviewing industry-funded studies. Institutions embracing single IRB review also benefit from more extensive knowledge and experience the single IRB develops over the course of the trial, as the single IRB examines it through the lens of multiple-institutions’ results, ethical concerns, and local contexts. Importantly, the single IRB also develops a more informed and effective position with the sponsor by advocating the views of all the institutions reliant on it, and the single IRB often becomes a consultant to the sponsor.
Today, single IRB review is the new norm. Roughly three quarters of institutions that have their own local IRB already rely in part on review by an outside IRB within their IRB Ecosystem8. The National Cancer Institute (NCI) has played no small role in this shift, requiring single IRB review for cooperative studies since 20149.
The 21st Century Cures Act strongly encourages single IRB review, and more recently, a mandate requiring single IRB review for all US sites conducting NIH-funded multicenter clinical trials has become part of official NIH policy10. Beginning January 25, 2018, NIH will no longer tolerate multiple re-review of the research they fund, with very few exceptions. This will affect thousands of studies. This mandate will extend to virtually all other federally-funded multicenter clinical trials in three years, as stated in the recently-revised Common Rule.
In the current state, the US government has already been heavy-handed about single IRB review, relieving industry of this burden and removing the attendant risk of upsetting people at institutions conducting federally-funded research. Thanks to new policy and new rules, the few organizations that have thus far chosen to eschew single IRB review now understand that they have no choice but to accept single IRB review if they want to participate in government-funded research. First the NCI, then the entire NIH, and now the Common Rule have changed the facts on the ground. The ground is now fertile for industry to do the same.
In retrospect, it seems somewhat surprising that the government has taken the lead, and industry is lagging. Perhaps it is due to the competitive landscape within which sponsors find themselves? Government agencies and lawmakers-which do not face this competition-made this decision based upon a desire to reach endpoints more quickly, to inject greater consistency in research oversight, and on the belief that it will help save money and improve people’s lives. Of course, industry has similar goals.
When sponsors and CROs give institutions a choice of local vs. central review, it results in inefficiencies, inconsistencies that may be challenged in hindsight, lack of consistent focus on patients as the primary customer of Informed Consent language, and in many cases, poor quality of multiple IRB reviews. These results will not change as long as a choice is available.
There is little doubt that some institutions will push back on single review, at least at first. In fact, before the government issued single review mandates, it sought public input as required when making new rules. There was indeed significant public comment, and there was a paucity of persuasive pleading in favor of multiple IRB re-review. Likewise, the 21st Century Cures Act underwent many rounds of revision before becoming law. Many did not see how their individual ethical standards could be represented by an outside single review IRB. Today, these arguments have largely faded into the background, and institutions that have adopted the single review solution have experienced the benefits. It is only a matter of time until the majority of institutions come to understand the quality benefits, the administrative savings and industry-partner-of-choice status that moving away from local IRBs will bring. As the industry shifts, and ultimately, when sponsors stand firm on single IRB review, it will be accepted. Perhaps grudgingly at first-at a few institutions-but accepted nevertheless.
So what are we waiting for? There is no better time than now for biopharma and medical device industry leaders to follow the NIH lead and the recommendation in the 21st Century Cures Act, and set a date-certain, after which local IRB review of multicenter clinical trials will no longer be supported.
Dawn M. Furey is Executive Director, Head of Global Operations, Global Clinical Trial Operations at Merck & Co.
Stuart Horowitz is President, Institutions and Institutional Services at WIRB-Copernicus Group.
References
1. The terms single review and central review are used interchangeably here.
2. There are approximately 3,000 registered IRBs in the US. As of this writing, approximately 7% are accredited. http://aahrpp.org/learn/find-an-accredited-organization
3. http://www.wcgclinical.com/institutional-irb-performance-2015-optimizing-benchmarking-report/
4. https://admin.share.aahrpp.org/Website%20Documents/2016%20All%20Organizations.pdf
5. http://www.sairb.com/services-technology/turnaround-times/
6. http://www.trials.novartis.com/en/clinical-trials/us-oncology/oncology/signature/
7. http://www.quintiles.com/microsites/investigators/precision-enrollment
8. Horowitz, S and Eibeler, C. An IRB Ecosystem Improves Human Research Protection Programs. New Perspectives on Healthcare Risk Management, Control & Governance; Fall 2016, Vol. 35 No. 3, p52-56.
9. https://ncicirb.org/cirb/default.action
10. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-16-094.html