Clinical Trial Agreement Negotiations
There are opportunities to make the negotiation process more efficient and reduce timelines.
Managing clinical trial agreements with hospitals and investigators can be quite a challenge, especially when dealing with a large multi-country study. Many different parties are involved in the process and you have to deal with a variety of laws and regulations, languages, cultures, social and historical backgrounds, and different local or even regional customs.

Lack of knowledge and experience in the above-mentioned areas may result in a substantial delay in the contract management process and, as it appears, in the overall clinical trial process.
In surveys conducted by CenterWatch, investigational sites indicated that contract negotiations were considered to be among the top delaying factors when performing a clinical trial, both in Europe and the United States.1 Interestingly enough, when asked in the CenterWatch survey about solutions to avoid delays, none of the participating sites mentions improvement of the contract management process, which could be perceived as a "necessary evil" rather than an opportunity for process improvement and eventually for an increase of quality and reduction of costs.

ERIC AUDRAS/GETTY IMAGES
Since there is not much data available on the general experience on and understanding of this topic from the clinical research industry (i.e., pharmaceutical companies, biotech companies, and contract research organizations), a survey was conducted by Salvius Legal and Applied Clinical Trials to gain insight into the industry.
Although budget negotiations are related to the contract negotiations with the sites, and thus fall in the category of potential delaying factors in the clinical research process, the focus of the survey and this article shall remain limited to the actual contract negotiation of the legal terms.
The survey
The survey was conducted by sending an e-mail to subscribers of Applied Clinical Trials inviting them to fill out the questionnaire online, which resulted in 534 completed questionnaires. The respondents consist of a well-balanced mix of representatives, mostly from the clinical departments of companies ranging from small to very large CROs, pharmaceutical, biotech, and other companies or organizations.
Half of the companies and organizations are located in a single country, the other half in more than one country; however, 65% of the respondents operate in multiple countries across the globe. The group of respondents consists overall of a very solid representation of parties in the industry dealing with clinical trial agreements at an international level.
Site contracting: a perspective
Given the current economic situation and cost developments in the industry, it seems only appropriate to start with a short reflection on the relationship between clinical trial agreements and costs. Clinical trial agreements potentially influence the costs of a sponsor of a clinical trial in several ways. First, a poorly drafted and/or negotiated agreement can create legal risk exposure, which in turn could result in unexpected financial liabilities. Second, inefficiencies in the negotiation process can result in unnecessary additional costs associated with extra man-hours. Third, and possibly most serious, troublesome contract negotiations may delay a clinical trial and, in effect, delay the marketing of the investigational drug, resulting in severe loss of profit for a sponsor.
Clinical trial agreements are generally considered low-risk agreements, presumably because parties operate in a strongly regulated environment and the framework and requirements regarding the performance of the clinical trial are for a large part set in the protocol. In addition, parties have a scientific and ethical reputation to uphold, and therefore are not very likely to agree to terms that may jeopardize such reputation.
However, site agreements in which reimbursement clauses were not properly drafted do exist. Consequently, hospitals were able to claim costs that are not a reasonable responsibility of the sponsor to reimburse. Additionally, and often even more damaging, poorly drafted agreements may result in inadequate confidentiality and intellectual property clauses. If, for instance, a hospital is under no obligation to ensure that its study staff comply with the confidentiality obligations in the agreement or transfer any rights to the study data and inventions potentially arising from the trial to the sponsor, a serious impact on the sponsor's competitive edge could result in a considerable loss of profit.
The above should be put into perspective: If there is an opportunity in the agreement to "sabotage" a clinical study, it does not mean that this will actually happen. Investigators and hospitals are usually not focused on finding openings in the agreement to the disadvantage of the sponsor. Also, if there is a breach or problem, the parties will usually be more interested in settling in good faith, in order to avoid negative publicity or the risk that future collaboration will be severely jeopardized.
Proper negotiations
Notwithstanding the above, 9% of the respondents of the survey indicated that their respective companies have been in a situation where damages were caused to their company or other parties involved in the clinical trial because the contents of the site agreement were not properly negotiated and over 6% indicated that their company has been involved in a legal suit or action in which the contents of the clinical trial agreement was considered crucial for settling the suit or claim. Although it is clear that serious issues arising from poorly drafted site agreements are rather an exception than the rule, the results of the survey indicate that these exceptions should not be neglected.
Inefficiencies in the site contracting process have an influence on the costs of a clinical trial as well. Insufficient training, poor communication skills, and unclear processes can all result in drawn out communications between the parties or a duplication of work. Too many hours spent on a task and unnecessary delays are the result.
As mentioned, sites experience the contract negotiations as a top delaying factor. From the survey it appears that industry representatives also perceive it as the most important cause of delay in the clinical trial process (Figure 1). Most of the respondents indicated that contract negotiations usually take more than six weeks, but a large part also indicated longer than 12 weeks, and some even more than four months. Whether the contract negotiation period actually causes a delay will ultimately depend on how long other clinical trial start-up activities are taking, since contract negotiations usually run in parallel. However, it clearly is the perception and experience of the parties involved that this part of the clinical trial process takes longer than it should.
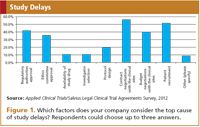
Figure 1
So why doesn't this part of the clinical trial process get the attention it deserves? Maybe the required experience and skills—legal, operational, and organizational—are often underestimated. In order to ensure good quality, consistent, and quick site contract negotiations, it is important to put in place clear processes, good-quality documents, and well-trained and experienced staff with solid communication and organizational skills. Contract negotiations will always be a part of the process that most operational staff consider as inconvenient but necessary, and even the best contracts management team cannot prevent negotiations taking a certain amount of time. However, negotiation times can be reduced by following the right approach and acknowledging what is required to achieve this.
When looking at costs associated with delays of a clinical trial, it becomes even more urgent to consider whether your company is taking sufficient action to avoid such delays. Everybody working in the industry is aware of the enormous costs of developing a new drug. Costs for delay of a clinical drug trial are estimated to be on average $1.3 million per day.2
Being aware of this fact makes it easy to conclude that each day saved on contract negotiation time, may save a pharmaceutical or biotech company substantial amounts of money.
Reasons for delays
Country- and industry-specific knowledge. Delays often occur because of a lack of knowledge on applicable local laws and regulations, as indicated by the respondents of the survey. There are situations where lawyers insisted on trying to negotiate certain clauses in the agreement that were an exact copy of what was stated in the law or that would be overruled by imperative local legislation.
The different cultural backgrounds and local customs are also factors to be taken into consideration for the planning and approach of the negotiations: it is for instance helpful to know that in the months of July and August, it can be very difficult to progress negotiations in a majority of the countries of Southern Europe, because of the long vacation period.
Besides the local specifics, a contract manager should also be fully aware of the actual clinical trial process and the differing risks involved with different types of trials. Such awareness is necessary so that a proper risk assessment can be made and a pragmatic approach applied. If a certain situation is very unlikely to occur in a clinical trial, and even if it occurs, presents minor disadvantages, it is not necessary to insist on language that deals with the situation. Also, a contract for an observational trial, for instance, has a different risk level compared to a contract for an early phase clinical trial.
Resources. The respondents of the survey indicated that site agreements are usually dealt with by internal staff of the concerning company: internal lawyers, internal contract managers, the clinical department, but also administrative staff (Figure 2). Legal staff usually deal with the review of actual legal terms. Contract management, administrative staff, and sometimes members of the clinical department review agreements to the extent such relate to administrative changes or are based on pre-agreed fallback provisions, and are responsible for tracking and organizing the agreements. The clinical team is usually serving as the direct liaison with the hospital and the investigator.
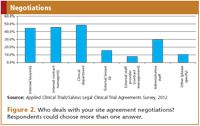
Figure 2
It is clear that where site contracting is not managed properly it may result in unnecessary risks, inefficiencies, and delays. Therefore, it seems only logical to ensure that for this part of a clinical trial, sufficient, qualified staff, possessing the required skills and experience, are made available. This is apparently a challenge, since the respondents of the survey indicated it is not easy to find qualified staff (Figure 3). However, the workload in this area can fluctuate considerably, which makes it difficult to always have sufficient and trained staff available.
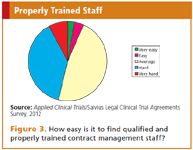
Figure 3
It is in that sense interesting that only a few respondents of the survey use external resources for dealing with site agreements, since it could offer flexibility and the required expertise when needed. It could be the case that parties have the idea that contracting for such external expertise is too expensive (the average hourly rate paid for this service seems to be between $200-$300). Granted, if the external party delivers a poor service—rigid lawyers that do not have the industry experience and do not share the same sense of urgency that is usually associated with these agreements—it can add substantially to the costs without providing the required benefits. However, if the external party can truly contribute to the improvement of the timelines, efficiencies in the process, and quality of the contracts, it can save money for the company.
Either way, qualified contract management resources are worth investing in, whether contracted or employed. The respondents of the survey seem to realize this; they opine that involvement of contract management specialists with a solution-oriented, pragmatic approach and with experience in the industry could improve the process.
Clear processes, training, and communication. Well written contract templates, logical processes, and consistent, good quality contract reviews are important if a company wishes to reduce risk, inefficiencies, and delays; also if a company desires some control over the documents it signs and has an interest in maintaining the image of a professional and reputable organization.
Therefore, besides the availability of quality resources, it is important to ensure that clear processes are established and proper contract management tools are available. A tracking program, fallback provisions, a "previously approved language" list, and review guidelines that clearly define the responsibilities of the different parties will contribute to the above described objectives, as also indicated by the respondents of the survey.
Of course the processes and tools will only work if the parties involved are properly trained. The training should also include good communication guidelines and skills; the lack of proper communication is considered as a bottleneck in the contract management process by the respondents of the survey (33%) (Figure 4).
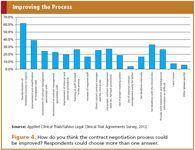
Figure 4
In order to evaluate the processes and identify bottlenecks and stagnations in the process it is important to track the progress of the negotiation. There are many reasons why delays occur, and many of them can be avoided if the cause of the delay is known. Lack of clarity on who is responsible to move things forward at a certain stage, too high a workload of the parties responsible for the negotiation process, or a contract slipping through the net can all create delays, but if identified in time while tracking the progress and followed-up by the correct action, it will be possible to minimize the consequential delays. Keeping metrics of the turn-around times can also assist with setting the right expectations with the different parties involved, and help with realistic planning.
Apparently, however, many companies do not have an official, solidly working tracking system or follow-up processes in place. The survey shows that most of the respondents are not using an official tracking system but are using simple methodology such as an Excel sheet. Not quite half of the respondents indicated that they keep metrics on turn-around times. There is obviously still room for improvement in this area (Figure 5).
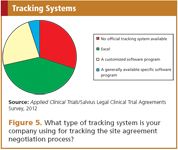
Figure 5
Country and site selection. A large treatment-naïve patient population, a well-organized health system, low costs, the availability of certain key opinion leaders, and other factors influence the decision to select a country to participate in a clinical trial. From the survey it appears that troublesome contract negotiations can also be made part of that list: 30% of the respondents indicate it can influence their decision not to select a country.
It is important for the decision maker to know the actual reason for difficult negotiations in that country. It could be the case that the staff dealing with these agreements are simply not familiar enough with the local laws, regulations, and practices. Countries that are perceived as most "difficult" by the respondents of the survey are the United States, France, Spain, Italy, Russia, and Poland. The European countries listed are typically countries where one could save valuable time were familiar with the local requirements. In such cases, it is worthwhile to ensure proper training of contract management staff, so that the decision to select a country can be based on other factors that are more difficult to influence.
Demanding country-specific procedures, laws, and regulations, or bureaucracy at the sites are harder to avoid. In such cases, it might be interesting for local organizations that promote clinical research in their country to use their influence in order to help adjust local laws and regulations, to stimulate more efficient local procedures, and to facilitate education and support of the relevant site staff and/or investigators.
When selecting sites, a vast majority of the respondents (80%) state that previous bad experience with the contract negotiations at the site influences their decision to work with that site in the future. There can be reasons to select a site despite a poor reputation with respect to contract negotiation timelines, for instance the availability of a key opinion leader, but the results of the survey imply that timelines are eventually a major decisive factor (Figure 6).
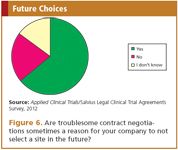
Figure 6
Communication with the sites. The results of the survey show that the general perception in the industry is that hospitals or investigators participating in a clinical trial often cause delays in the contract negotiation process due to rigid hospital lawyers and a lack of a sense of urgency at the site. The industry perceives turn-around times to be unnecessarily long.
Bureaucracy and lack of understanding are difficult to avoid; however, there are still ways to influence the progress of the negotiations. Setting deadlines with the sites seems self-evident, but is often not done. Another way to speed up the process is to provide a solid explanation and background information on difficult topics to clinical sites. If necessary, instead of a member of the clinical team trying to negotiate with the site on the basis of comments of the lawyer or contract manager, the latter could get in touch with the site directly to solve any outstanding items.
Training, education, and creating awareness both at the site and with the contract managers could in the long term contribute to a solution for this delaying factor.
If at the beginning of a negotiation the topics that are usually difficult to negotiate are known, it provides the opportunity to anticipate and pre-empt such problems. Suggesting clear and fair language and providing additional explanation on difficult or sensitive topics can make a difference. According to the outcome of the survey, the most difficult topics to negotiate in site agreements are terms relating to budget and payment, intellectual property and inventions, indemnification and liability, publication, insurance, and applicable law and jurisdiction.
Not only can the sites find clauses on these topics difficult to deal with, also contract managers without a thorough legal background or sufficient experience find these topics challenging to negotiate. Training on these specific topics would help staff draft clear and more acceptable clauses, understand them, and explain the language and its rationale to the site.
Templates. The template, as a starting point of the negotiations, can have a tremendous influence on the speed and success of the negotiation process. A complicated and unclear template makes hospitals and investigators suspicious of what they are asked to sign and will create a reluctance to review the agreement and to agree on the terms. A well-structured, straight-forward template on the other hand, will inspire confidence and trust with the hospitals and investigators. This obviously will help speed up the process of getting the contracts agreed and signed.
For a sponsor of a clinical trial it might also be interesting to know whether it will be more (time and cost) efficient to use its own standard templates or the templates of the CRO that it has contracted to perform the trial and that is actually responsible for the negotiations of the agreements. A sponsor may benefit from using its own templates if it has conducted previous studies with the involved sites on the basis of the same template and, moreover, coming from the "sponsor," the templates may be more easily acceptable, due to increased authoritative value. In addition, for a large organization it is helpful to achieve some consistency in the documents that are signed with other parties, for better oversight and control. On the other hand, it could also be efficient to let the CRO use the template that it is familiar with, so that it will be easier and consequentially faster for it to negotiate the terms. Either way, there are disadvantages and benefits; eventually, the quality and suitability of the template may have a greater impact on acceptability than the party providing it.
Another option that is often suggested to save negotiation time is the standardization of the clinical trial agreement template. Respondents of the survey also indicated this as an important solution for cumbersome contract negotiations. In some countries, like the United Kingdom, the involved interest groups successfully introduced a standardized template for clinical trial agreements. The reason why it seems to work in the United Kingdom is that:
- The template is created jointly by the representatives of the parties involved in clinical research, which creates a solid basis for acceptance of the terms
- The template is of high quality
- Room is left for flexibility
If approached in the same way as in the United Kingdom, a standardized country-specific template can facilitate negotiations. However, a worldwide or continent-wide standard template will be difficult to achieve, since there will be too many local differences to overcome.
Translations. In many countries, site agreements need to be translated into the local language, sometimes only for information purposes, sometimes because it is a legal requirement or because the site is not willing to sign a contract in another language.
A CRO and a sponsor of a clinical trial often agree on a certain policy with respect to translations. Entire templates and larger pieces of text in an agreement under negotiation are usually translated by certified translators while small parts are often translated by local clinical staff into and from their native language. This approach is a pragmatic one; however, small words can make a considerable difference in legal meaning. Therefore, it might be prudent to consider using a certified translator in all cases except for purely administrative parts such as names and addresses.
Proper translations of the contracts are important not only because of the risk of committing to contents that one did not intend to, but also because unnecessary delays may occur. For example, a party may reject certain terms that would have been absolutely acceptable to that party, had they been properly translated.
The majority of the respondents to the survey indicated that they use certified translators for the translation of their agreements (almost 60%), but not even 30% seem to use translators with experience in the industry. In order to avoid the above-mentioned risks, it is important to make use of translation agencies that are very familiar with the terminology in the industry. For example "subject" may be translated to "topic" in the local language, although clearly a study participant was meant by the term.
Eventually it is important to create a balance between being pragmatic and proper risk management: the lawyer or contract managers can help decide which parts are important enough to run by a certified translator. Ensure the translator has experience in both industry specific and legal terms.
Conclusion
Contract negotiations with hospitals and investigators are time-intensive. However, there are still many areas that present opportunities to make the negotiation process more efficient, reduce timelines, and at the same time maintain a proper risk and quality standard.
Realizing the potential high costs involved with unnecessary risks, inefficiencies, and (especially) delays, and given the situation that CROs, pharmaceutical, and biotech companies are increasingly forced to make their decisions on the basis of cost efficiencies, it seems only evident that it is worthwhile to invest in experienced and qualified staff and/or to contract external resources that possess the required experience and skills. Improving clinical trial agreement negotiations is an opportunity to be seized.
Myrthe Rijswijk-Trompert is CEO and Senior Legal Consultant at Salvius Legal, Salvius Legal BV, Karpervijver 12, 3703 CJ Zeist, The Netherlands, e-mail: mrijswijk-trompert@salviuslegal.com.
References
1. Mary Jo Lamberti, ed., "State of the Clinical Trials Industry A Sourcebook of Charts and Statistics" CenterWatch (2008),
2. C. P. Adams and W. Brantner, "Estimating the Cost of New Drug Development: is it Really 802 Million Dollars?" Health Affairs, 25 (2) 420-208 (2006).

FDA Expands Farapulse PFA Approval to Persistent AF After Strong ADVANTAGE AF Trial Results
July 7th 2025Boston Scientific’s Farapulse Pulsed Field Ablation System is now approved for treating persistent atrial fibrillation, following 12-month data from the ADVANTAGE AF trial showing strong safety, high freedom from AF, and no major complications.
Unifying Industry to Better Understand GCP Guidance
May 7th 2025In this episode of the Applied Clinical Trials Podcast, David Nickerson, head of clinical quality management at EMD Serono; and Arlene Lee, director of product management, data quality & risk management solutions at Medidata, discuss the newest ICH E6(R3) GCP guidelines as well as how TransCelerate and ACRO have partnered to help stakeholders better acclimate to these guidelines.
FDA Grants Priority Review to Merck’s sBLA for Winrevair After Early Success in ZENITH PAH Trial
July 2nd 2025Merck’s bid to update Winrevair’s label advances with FDA priority review, backed by Phase III ZENITH data showing a 76% reduction in major morbidity and mortality events in patients with pulmonary arterial hypertension.