Master Protocols: Implementing Effective Treatment Adaptations in the Randomization
It is unrealistic to include infinite adaptations in an IRT system, thus identifying the optimal level of adaptations requires examination of the study’s characteristics and planning phase considerations.

Master protocols are innovative clinical trials designed with multiple sub-studies, subgroup-populations, and subprotocols. Their benefits include increased flexibility and efficiency in drug development, possibility for shared control arm(s), centralized data-capture systems, and patient centricity.1
Master Protocol Types include:1
- Basket: single investigational arm studied across multiple disease populations (Figure 1a)
- Umbrella: multiple investigational arms across single disease population (Figure 1b)
- Platform: may have common basket/umbrella attributes, multiple phases/treatments/diseases / populations/sponsor companies (Figure 1c)
Master Protocol Types
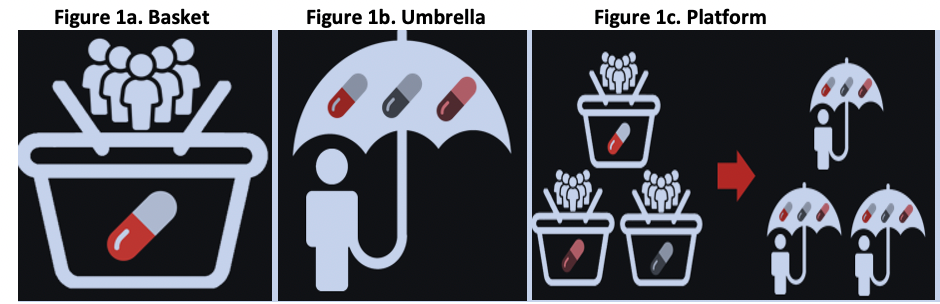
Source: Almac Clinical Technologies
Traditional studies are one dimensional, focusing a single investigational arm within a single disease population2. If any adaptations are incorporated, they would likely be straightforward. Whereas Master Protocols (especially platform designs) are usually perpetual, inherently more complex than traditional trials3, and typically require adaptations to occur on multiple dimensions. To handle their complexity and multidimensional randomization adaptations, a sophisticated Interactive Response Technology (IRT) system is necessitated. Because of their complex nature, increased collaboration during the planning phase is required compared to standard trials.3,4,5,6
Introducing new treatments (e.g., experimental/investigational /control arm), as well as discontinuing existing treatments, are routine adaptations in master protocols.5,6,7 As highlighted by Woodcock and LaVange,6 a main hallmark of master protocols is innovation when randomization adaptations facilitate adding new treatments with minimal disruption. Thus, it is recommended to design the IRT’s randomization to support seamless treatment adaptations. This paper will focus on both the important study-specific considerations for determining appropriate flexibility levels, as well as treatment adaptations within randomization.
Consideration 1: What are the Multiple Dimensions (Subgroups)?
The evaluation’s first step is establishing the study-specific subgroup definition(s). For instance, a master protocol may comprise of subprotocols that can each introduce new treatments. Within each subprotocol, additional subgroup levels may exist (e.g., stratification, cohorts, biomarkers) with differing treatments. Thus, a study-specific subgroup definition may be the combination of subprotocol and cohort. For illustration, this paper defines it generically as ‘subgroup’. However, in reality, it is best to use terminology that is meaningful to the specific master protocol.
In practice, a real-time treatment adaptation IRT feature is needed where a designated user can manage randomization adaptations (e.g., opening / closing treatments) within each subgroup. Figure 2 shows how this adaptation feature is configured for each subgroup with independent settings (treatments, statuses, and ratios). In conjunction, a separate randomization scheme is associated to each subgroup and mapped based on each subgroup’s current settings.
Figure 2. Treatment Management within Subgroups
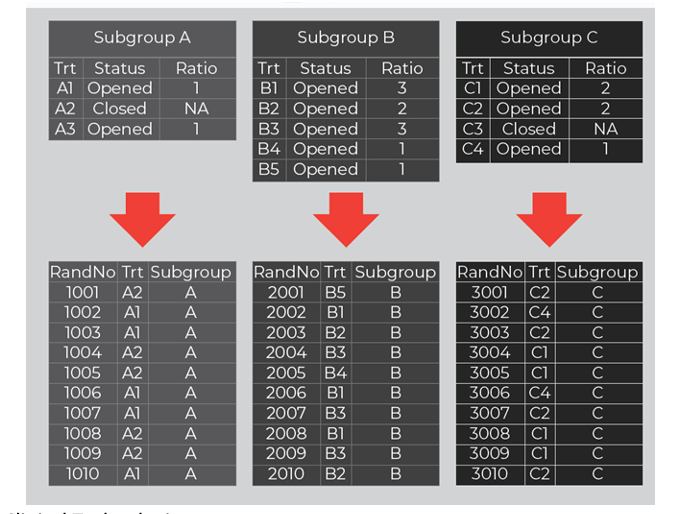
Source: Almac Clinical Technologies
Consideration 2: How Many Treatments?
The master protocol’s initial version typically describes the treatments included at the study’s start for each subgroup. Some future treatments may be known, but most are generally unknown. Depending on the methodology (e.g., list-based, probability-based), the IRT’s randomization may incorporate placeholders. If placeholders are required, the study team needs to determine a reasonable number that provides sufficient overage.
Consider how many:
- Initial treatments
- Expected new treatments throughout the study
- Treatments can be open at a given time
Including too many placeholders could significantly elevate start-up time and costs. With master protocols, it may be challenging to predict the future, in feeling comparable to the Goldilocks Dilemma (see Figure 3). Effective collaboration will lead to realistic estimation for optimal flexibility.
Figure 3. Goldilocks Dilemma: How Many Placeholders?
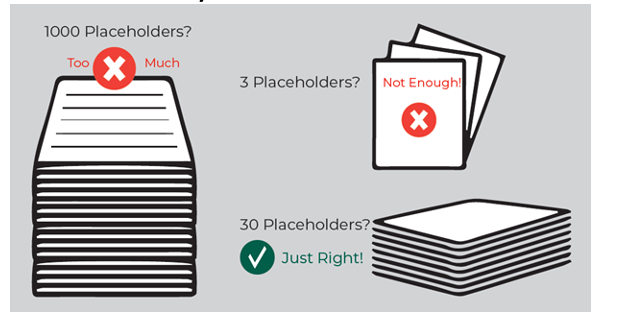
Source: Almac Clinical Technologies
Consideration 3: Treatment Ratio Requirements?
The master protocol’s initial version usually defines each subgroup’s ratio(s) applied at the study’s start. Some protocols may keep fixed ratio(s) for the entire study (i.e., always equal allocation ratio).
While others may require ratio or probability adjustment (e.g.,):
- Target for all treatments need to hit at the same time
- Reprioritization of treatment(s)
- Ratio adjustment due to shared control group5,6
- Favor treatments based on subject responses (e.g., Bayesian Response Adaptive Randomization)2,4,5,8
Ratio adjustment parameter considerations may include:
- Protocol requirements / restrictions
- Maximum ratio weight (e.g., max.=5, 5:1, 5:1:1)
- Total maximum ratio weight (e.g., total sum of weights <= 10)
- Full flexibility (e.g., probabilistic assignment)
For implementation, the treatment adaptation feature can be structured for a designated user to enter each treatment’s ratio weight (probability or target, etc.), and the randomization designed to accommodate flexible ratios (e.g., list with placeholders, dynamic block sizes or probability-based algorithm).
Consideration 4: Will Site-Readiness Differ for New Treatments?
Modifications, such as introducing a new treatment, require Institutional Review Board (IRB) approval before implementation.1 The new treatment may also include a new medication type. The timing of IRB approval and medication availability can often differ across sites; thus, sites may not be ready for the new treatment at the same time. If site-readiness can vary, randomization can be adapted based on site-level approval of new treatment(s). New treatment(s) are included in randomization for approved sites, and not included for sites without approval.With this approach, approved sites can begin randomization to the new treatment without delay.
Consideration 5: Can Treatment Eligibility Vary?
For study inclusion, some master protocols may require subjects to be eligible for all treatments, while others may allow treatment eligibility variability. If eligibility can vary, the scale of ineligible subjects should be considered. If only a few ineligible subjects are expected, the randomization could assess eligibility at the individual level to prevent assignment for anyone ineligible. If ineligibility is expected on a greater level like an entire subgroup, then the adaptation feature can be designed to manage treatments (open / close) at that subgroup level. For example, biomarker negative subjects are ineligible for a biomarker-targeted treatment. That new treatment is closed for the biomarker negative subgroup and excluded from their randomization.
Consideration 6: Inclusion in Initial IRT vs. Amendment
Including infinite adaptations within the initial IRT is unrealistic. To determine which adaptations to incorporate initially versus later, evaluation should identify what is known, most likely, and completely unknown. For example, an initial master protocol consists of three subprotocols, each with a unique population. Within each subprotocol, new treatments can be added (or dropped) and ratios adjusted. New subprotocols can also be introduced. Sufficient information is known about the randomization adaptation needs for the known subprotocols. However, the randomization composition for any new subprotocols is unknown (i.e., may have different randomization methodology, stratification, cohorts, etc., from existing subprotocols). Therefore, the initial IRT contains the known subprotocols, but not any future subprotocols. Since there are too many unknown randomization factors in future subprotocols, any placeholder subprotocols may not be valid or used. It is more realistic to evaluate and include when the protocol is amended to specify the new subprotocol. The ultimate goal is to develop the initial IRT for routine adaptations and most likely possibilities.
Conclusion
To date, oncology trials have implemented the majority of master protocols.2,3,9 Due to oncology’s successes, interest is growing throughout non-oncology sectors.6 Given COVID-19’s prevalence, Natanegara et al (2021)7 explained that pandemic research would benefit from these adaptive designs for efficiency and robust decision making. Moreover, master protocol numbers are anticipated to rapidly increase over the upcoming decades.9 With the expected increase, Park et al. 20199 emphasized that more efforts are needed to improve awareness and training for the application of these innovative designs. This paper intends to contribute to these efforts with providing important planning phase considerations to ensure the master protocol’s randomization effectively allows for routine adaptations.
After successful evaluation and implementation, new treatment(s) can be introduced in the IRT randomization in real time. That is, executing adaptations (e.g., open / close treatments, adjust ratio) without needing to create a new randomization scheme for each protocol treatment amendment.While this is not the exhaustive list of considerations, it is imperative to understand that each study is unique and requires protocol-specific assessment. Likewise, Woodcock and LaVange (2017)6 conveyed that the complexity of adaptations can vary based on the master protocol’s objectives. While this paper has focused on randomization, new treatment on-boarding should also be addressed from the clinical operations and drug supply perspectives to ensure overall efficiency.
Compared to traditional one-dimensional trials, master protocol planning should begin earlier to account for stakeholder coordination, infrastructure requirements, and complex trial design elements.3,4,5,6 Thus, the IRT randomization evaluation should also start early so that sufficient examination and collaboration can transpire amongst stakeholders. The success of master protocols is attributed to how quickly treatment adaptations can occur. If this collaborative investigation does not happen, this can lead to the development of an inefficient framework and result in slow adaptations. Effective collaboration is key to successful implementation and efficient adaptations.
References
- FDA, "Master Protocols: Efficient Clinical Trial Design Strategies to Expedite Development of Oncology Drugs and Biologics Guidance for Industry (Procedural)," 2022. [Online]. Available: https://www.fda.gov/media/120721/download.
- D. Berry, "The Brave New World of clinical cancer research: adaptive biomarker-driven trials integrating clinical practice with clinical research.," Molecular Oncology; 9, p. 951–959, 2015.
- M. Redman and C. Allegra, "The Master Protocol Concept.," Seminars in Oncology, 42(5), p. 724–730, 2015.
- FDA, "Adaptive Designs for Clinical Trials of Drugs and Biologics Guidance for Industry," 2019. [Online]. Available: https://www.fda.gov/media/78495/download.
- L. Renfro and D. Sargent, "Statistical controversies in clinical research: basket trials, umbrella trials, and other master protocols: a review and examples.," Annals of Oncology, 28, p. 34–43, 2017.
- J. Woodcock and L. LaVange, "Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both.," The New England Journal of Medicine, 377, pp. 32-70, 2017.
- F. Natanegara, N. Zariffa, J. Buenconsejo, R. Liao, F. Cooner, D. Lakshminarayanan, S. Ghosh, J. Schindler and M. Gamalo, "Statistical Opportunities to Accelerate Development for COVID-19 Therapeutics," Statistics in Biopharmaceutical Research, p. DOI: 10.1080/19466315.2020.1865195, 2021.
- W. Barry, C. Perou, P. Marcom, L. Carey and J. Ibrahim, "The Use of Bayesian Hierarchical Models for Adaptive Randomization in Biomarker-Driven Phase II Studies," Journal of Biopharmaceutical Statistics. 25(1), p. 66–88, 2015.
- J. Park, E. Siden, M. Zoratti, L. Dron, O. Harari, J. Singer, R. Lester, K. Thorlund and E. Mills, "Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials. 20:572, https://doi.org/10.1186/s13063-019-3664-1.," Trials. 20:572, pp. https://doi.org/10.1186/s13063-019-3664-1, 2019.
Getting IRT Right – Part 2: Study Drug Allocation and Supply Management
September 15th 2023In an earlier article, we reviewed the randomization risks that could arise if an interactive response technology (IRT) system isn’t designed and/or implemented correctly. Here we address the consequences that trial sponsors could face if their IRT system isn’t adequately designed to handle the many and often complex drug allocation and trial supply aspects of their clinical trials.