Taking eCOA Delivery Off the Critical Path to First Patient In
Streamlining electronic clinical outcome assessment and patient-reported outcome processes can aid in decreasing timelines.
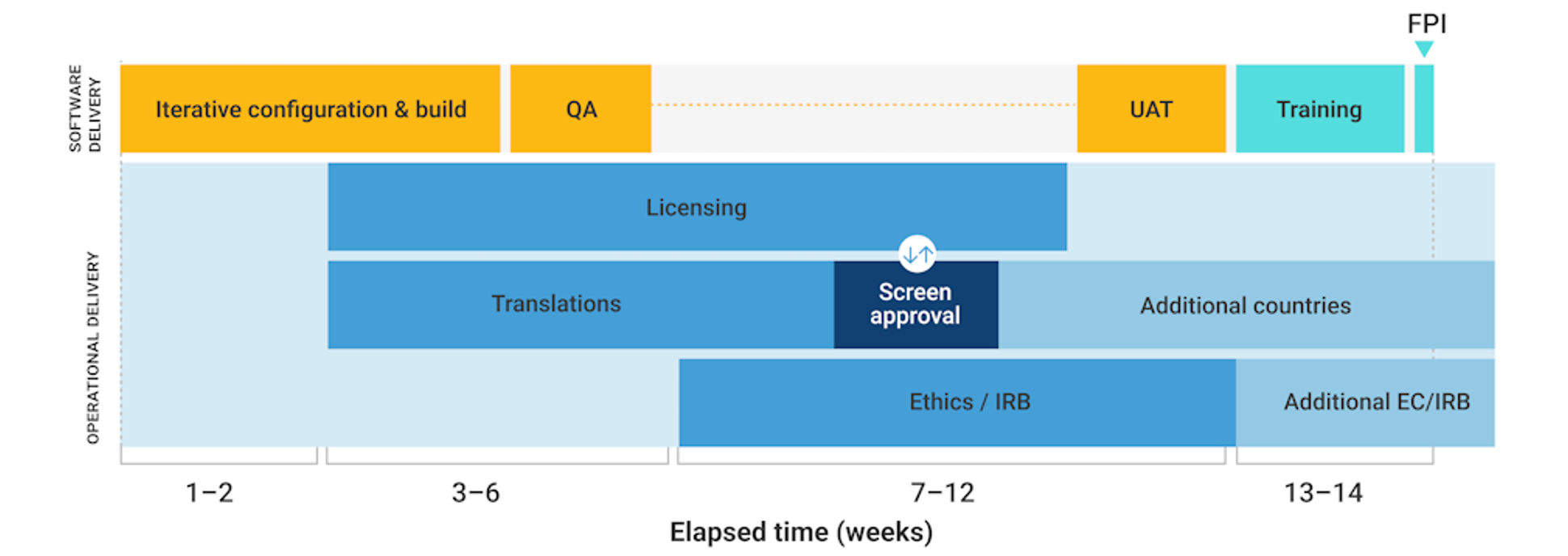
Meeting the first patient in (FPI) deadline is a critical milestone for study teams who must first implement various systems and processes as part of study startup. Despite other systems typically being ready on time, the delivery of electronic clinical outcomes assessments (eCOA), including electronic patient-reported outcomes (ePRO), often lags, taking up to fourteen weeks for a typical study (see Figure 1) and even longer for complex study designs.
Figure 1: Historic eCOA implementation timelines
Source: Veeva

The lengthy timelines put FPI milestones at risk and frustrate study teams. Over the past decade, software developers have focused on speeding delivery timelines by introducing agile development methodologies, configuration instead of customization, and reusable libraries of assessments to reduce software build times. Still, meeting FPI is more challenging than ever.
Despite the software advances to accelerate build times, operational processes such as licensing, translations, user acceptance testing (UAT), and ethics submissions remain largely unchanged (see Figure 2) as eCOA remains on the critical path to FPI. There is a pressing need for change to improve these processes and timelines, and simplifying instrument licensing and translations is an essential first step.
Figure 2: Current eCOA implementation timelines
Source: Veeva
Streamline instrument licensing and translations
Instrument licensing is arguably the most challenging aspect of eCOA delivery. Agreements are executed for each study and language, and authors are often individuals or small businesses with little understanding of eCOA technologies. The authors focus on retaining instrument validity, but the process is frequently long and arduous. Most instruments originate from paper, so having limited resources and a lack of technical expertise for transferring to electronic capture makes the licensing process a significant hurdle.
The current approach is unsustainable. A scalable process is necessary to accelerate licensing without increasing the burden on authors. eCOA instruments are often validated for every study and vendor, but the screens used by trial participants are, by definition, almost identical.
By supporting instrument authors in developing standard electronic versions that can serve as a blueprint for vendors, the industry can reduce some of the effort required for licensing. Moving away from code libraries and adopting a fully reusable library can remove the need to validate instruments for every study or reduce it to a single confirmatory review.
Similarly, for translations, the same PROs and languages are being translated repeatedly using time-consuming manual processes. Having standard licensed instruments and translations ready for eCOA providers would significantly accelerate timelines.
The responsibility is not just on authors and eCOA vendors, sponsors also have a role to play. By working with experts and starting licensing earlier, such as when contracting CROs, agreements could be in place before eCOA vendor selection so that source files are immediately available for implementation.
Increase user acceptance testing (UAT) efficiency
There is often confusion regarding who is responsible for UAT, with some sponsors incorrectly assuming the eCOA vendor should conduct it. However, sponsors or their specialist representatives should conduct independent UAT to ensure assessments are done separately from validation activities. Although large biopharmas typically have established UAT processes, smaller companies may lack the knowledge and resources, leading to UAT being treated as an afterthought.
As UAT is the last stage before the study is released, it is always on the critical path. Although plans may have allowed two weeks for UAT, it is often reduced to a few days, resulting in issues getting taken into production and leading to urgent change requests.
Many of these challenges arise from planning UAT sequentially during the build process. With an agile approach, UAT can be considered at the outset, enabling early review and feedback to ensure the build is progressing in line with requirements. This ensures the sponsor and vendor are ready and reduces the likelihood of major issues in the final testing. Developing libraries of reusable test cases and exploring alternatives to physical device shipments for testing can further streamline the UAT process.
Limiting UAT participation to essential groups is also imperative. A common error is using UAT as an opportunity for broader stakeholder review by individuals not part of the design phase. Such belated involvement leads to design-related queries that must either be disregarded or necessitate repeating builds, changes that can delay the study start. Providing visibility for key stakeholders throughout the build process allows them to contribute to the design beforehand so that any discrepancies between assumptions and the requirements specification are addressed early in the build process.
Figure 3: Three steps to increase User Acceptance Testing quality and efficiency
Source: Veeva

Rationalizing eCOA documentation for ethics review
Each ePRO implementation must be reviewed by the appropriate ethics committees (ECs) or institutional review boards (IRBs) before its release for use in a study. This includes a review of each screen in the local language, meaning project teams must spend days, sometimes weeks, capturing individual screenshots for the ethics pack. Ethic submission dates are often weeks before the ePRO system has been finalized and screens are available. Recognizing this burden, some systems automatically generate ethics review packs to remove the need to manually capture every screen.
The primary role of ECs/IRBs is to protect patients, but standardized, commonly used instruments pose little patient risk. Is a complete review of ePRO screens needed? Do they even have the resources to review every screen on every device? It is important to consider whether the evaluation of individual screenshots falls under the remit of ethics review or, instead, a quality control task to be performed by vendors and study teams.1
ICH guidance does not explicitly recommend ethics reviews for ePRO screenshots, and some IRBs now officially state on their websites that they do not require them. However, driving lasting change in life sciences is not easy. While there have been pockets of success in avoiding unnecessary ePRO screenshot submissions, replicating this globally has been challenging.
Think beyond the software
While software is at the heart of eCOA, the surrounding processes often present the greatest delivery challenges. Transitioning from a sequential operational delivery model to an agile approach, initiating planning and executing licensing, translations, and UAT preparation as early as possible can significantly accelerate timelines.
Figure 4: An opportunity for accelerated eCOA implementation timelines
Source: Veeva
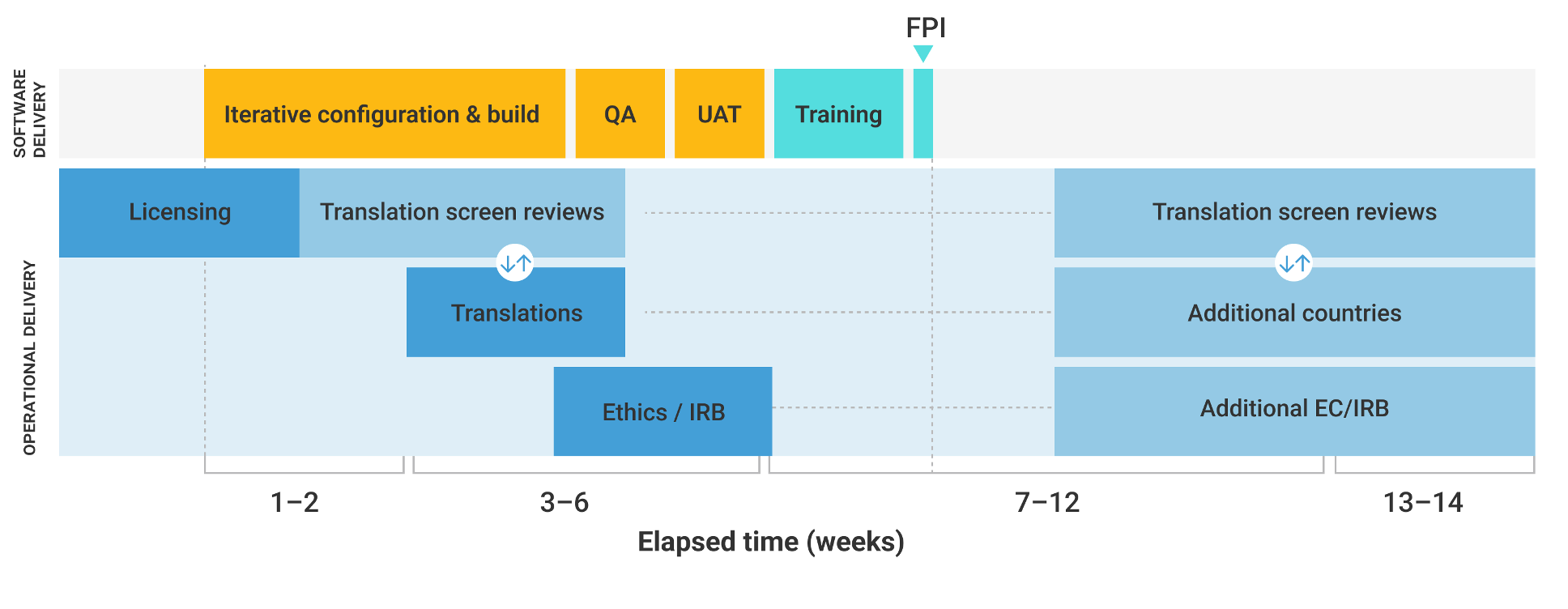
These steps, combined with greater standardization for electronic instrument versions and test scripts and automated documentation generation, can help to align operational activities with software delivery timelines (Figure 4). Addressing these issues collectively will deliver a smoother and more efficient process that ensures timely FPI and, ultimately, accelerates clinical research.
Tim Davis, vice president of strategy, MyVeeva for Patients, and Willie Muehlhausen, co-CEO & founder, Safira Clinical Research
Reference
1. Applied Clinical Trials, Demystifying Submissions of eCOA Documentation for Ethics Review: Are We Making Submissions More Difficult than Necessary?, 2020
Using Patient Reported Outcomes in Dermatology Trials
April 25th 2024In part 3 of this video interview with ACT editor Andy Studna, Melissa Mooney, director, eCOA sales engineering, IQVIA sheds light on the unique challenges of dermatology trials and how clinical outcome assessments can be implemented in them.