
Applied Clinical Trials
In contract research and contract bridge, the cards aren't as important as the people holding them.

Applied Clinical Trials
In contract research and contract bridge, the cards aren't as important as the people holding them.

Applied Clinical Trials
To better understand the complex world of licensing and enterprise software, consider a driving analogy.

Applied Clinical Trials
The EU offers up market advantages in exchange for pediatric trials.

Applied Clinical Trials
Federal agencies seek to curb redundant IRB procedures and encourage voluntary accreditation of research organizations.

Applied Clinical Trials
In the mid to late '80s, a tool called remote data entry was available which replaced double key data entry and paper case report forms (CRFs) at the clinical trial study site. When remote data entry (RDE) was used, the drug sponsor would provide a portable computer to the investigational site. The coordinator would collect study-related patient data, and then enter the data directly into the computer via the specially designed user interface of data entry screens. The electronic data would then be monitored. After data cleanup, a floppy disk with the site's data would be sent to the sponsor via an overnight courier service. These tasks would occur periodically during the course of the clinical trial.
Applied Clinical Trials
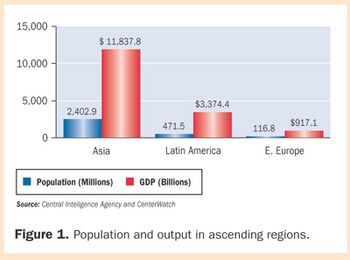
Clinical trials in developing nations-called "ascending markets"-are exploding. These regions offer large naïve subject populations, low operating costs, and increasingly stable testing infrastructures, but regulatory reform is really driving the growth. CenterWatch data show 20%?30% of clinical trials are being conducted in ascending regions.
Applied Clinical Trials
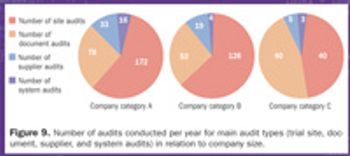
An extended survey was conducted on the topic of clinical quality assurance (CQA) benchmarking in pharmaceutical companies to gather new data.

Applied Clinical Trials
For all the promises of the impact of technology on clinical trials-from enhanced trial efficiencies to reduced time to market-very few specific examples have been published that clearly demonstrate a marked impact. This article provides just such an example, by examining the impact of electronic diaries (eDiaries) on the collection of Patient Reported Outcome (PRO) data in a large, pivotal Phase III trial evaluating a medication for the treatment of overactive bladder (OAB). We compare the results obtained using eDiaries to capture real-time data on voluntary and involuntary micturitions with those obtained from a Phase II trial that used paper diaries. The result: measurable subject protocol compliance and significantly reduced error variance, yielding increased study sensitivity and the opportunity to realize a significant financial savings in future trials.

Applied Clinical Trials
Datatrak International's Phase IV, Espicom's World Pharmaceutical Markets, and Keris, Inc.'s Vital Consent