Applied Clinical Trials
The expense of developing electronic submissions is manageable, even in a small company, and the benefits are great for both FDA and industry.

Applied Clinical Trials
The expense of developing electronic submissions is manageable, even in a small company, and the benefits are great for both FDA and industry.

Applied Clinical Trials
FDA plans to rewrite rules governing electronic records while offering new policies to encourage risk-based regulatory approaches to application review and inspections.
Applied Clinical Trials
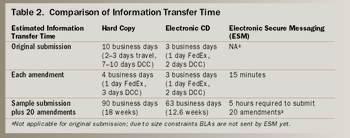
The economic consequences of inefficient work processes in clinical trials are significant. The average daily cost of drug development runs about $30,000 per day and rises by 10% to 12% per year. Development cycle times range from three to 12 years.1 In typical operational practice, the line management of a competitive firm strives to achieve the highest volume of successful new drug application (NDA) submissions (effectiveness) in the shortest practical time (efficiency). This combination reflects the business throughput of an organization.

Applied Clinical Trials
eClinical Trials: Planning & Implementation is a useful resource for those new to the industry as well as practitioners already involved in planning and implementing eClinical trials.

Applied Clinical Trials
Earlier this year, Applied Clinical Trials published "Clinical Monitoring: Answers to Questions about Good Clinical Practice," another excerpt from the book, Good Clinical Practice: A Question & Answer Reference Guide. Readers are referred to the July 2003 issue (pages 27-29) to read the full text of the reference guide introduction.

Applied Clinical Trials
European Research Commissioner Philippe Busquin tours Africa to bring attention to the plight of developing nations unable to afford modern medicines.

Applied Clinical Trials
Relatively few people are familiar with the software design process, but many have had their homes remodeled; the two processes are more similar than dissimilar.

Applied Clinical Trials
Interactive voice response systems are commonly used in clinical trials to manage the flow of trial medication supplies to sites and to manage the allocation of these supplies to individual subjects. Other advantages and uses include access to real-time information for trial managers, collection of diary card data directly from subjects, and as an aid to subject recruitment.1