An Examination of the Use of Patient Recruitment and Retention Tactics for Global Studies
In light of scant published data from sponsors and contract research organizations on site activation and recruitment challenges following the COVID-19 pandemic, the primary objective of this research is to revisit the results of recruitment and retention tactics in studies conducted in 2012 and 2019.
Image credit: Dzmitry | stock.adobe.com

Introduction
Patient recruitment and retention remains a pervasive challenge among pharmaceutical and biotechnology companies and contract research organizations (CROs) conducting global trials. Despite diverse tactics that have been used to recruit and retain patients, many have had variable impact.
A meta-analysis of 45 clinical trials found mixed results using traditional recruitment tactics. Use of opt-out rather than opt-in strategies increased recruitment as well as telephone reminders; however, other approaches, such as utilizing videos to provide a participant with clinical trial information or interventions with site staff recruiting potential study volunteers were inconclusive.1
Other studies using registries were found to be problematic as lists of potential patients generated by these registries included many ineligible patients due to inclusion and exclusion criteria not being listed on the registry, or stored elsewhere.2,3 In a more recent meta-analysis of 23 studies, some traditional tactics were found to improve recruitment, such as phone reminders, contacting potential patients with an opt-out instead of opt-in strategy, and informing patients about which treatment they would be receiving in the trial.4
Researchers, however, were unable to determine the impact from the use of delivery of trial information or degree of contact by investigative site staff with participants. The study did find, however, that online recruitment was more effective in half of the studies examined across a broad range of therapeutic areas and represented a lower cost for enrollee compared with offline recruitment defined as traditional in-clinic recruitment.4
More novel—virtual and/or decentralized—tactics have been the focus of published studies in more recent years. In published research examining 13 studies conducted between 2018 and 2022 on decentralized methods of recruitment and retention, advantages and success were found with recruitment and retention through the use of eConsent, wearable biomarkers, and virtual visits.5
The researchers acknowledge that more data are needed to compare metrics on recruitment and retention from decentralized trials with traditional trials.5 However, considering patient satisfaction and its relationship to increasing retention, another study found that both traditional and newer “convenience-enhancing” solutions increased patient satisfaction in clinical trials, ranging from childcare, and travel reimbursement to smartphone apps and wearables.
Traditional solutions included childcare and visits to their regular physician, while newer solutions included video conferencing and smartphone apps.6 Other studies have examined the use of social media as a recruitment tool and acknowledge its potential but caution its ethical implications.7 Recruiting from online networks and online communication that can potentially occur between and among clinical trial participants as well as privacy implications have effects that need to be addressed.7-9
In prior Tufts CSDD research conducted in 2019 among sponsor companies CROs, traditional recruitment tactics were more commonly used, including patient brochures and letters, flyers, and other print materials.10,11 Visit guides were reported as the most used retention tactic in the 2019 study.
Similarly, traditional tactics were also used in a prior study carried out in 2012.12 As these two studies were conducted pre-pandemic and before the rapid acceleration of remote and virtual solutions for clinical trials, increased usage of non-traditional approaches and social media were investigated further in this current study.
The uptake of digital tools, including use of telemedicine, online methods of recruitment; eConsent; use of patient apps for data collection and other patient-centered innovations, allow for potentially increased enrollments by improving access to clinical trials, reduced patient burden, and increased patient retention.13 Digital recruitment and retention may also help address enrollment challenges, which allow for more participant outreach and convenience for study volunteers in not having to travel to sites.14
In light of scant published data from sponsors and CROs on site activation and recruitment challenges following the COVID-19 pandemic, the primary objective of this research is to revisit the results of recruitment and retention tactics used in the previous Tufts CSDD studies conducted 2012 and 2019,10-12,15,16 Researchers analyzed data gathered from 17 pharmaceutical, biotechnology companies and CROs and on global recruitment and retention tactics across 98 studies.
Methods
In early 2023, Tufts CSDD collaborated with a working group of 14 top pharmaceutical companies and three large CROs on a study of patient enrollment and site activation benchmarking metrics. Companies completed a data collection instrument comprised of an Excel spreadsheet.
The data collection instrument was modified from a 2019 study that examined data from 87 global clinical studies primarily from phases II and III with early phase studies included in the sample but analyzed separately.11 An earlier study conducted in 2012 examined 151 global clinical trials primarily from phases II and III.12 Comparisons among the three studies were made where possible given the modifications in the data collection process over the 11-year time period.
In this current study, each working group company contributed between five and 10 Phase II or Phase III studies across five therapeutic areas, including oncology, central nervous system/neuroscience, inflammatory, vaccine, and cardiovascular/metabolic that randomized the first patient between 2018 and 2023. Studies could be ongoing but needed to have completed enrollment.
Any studies being conducted by CROs on behalf of pharmaceutical companies participating in the group were excluded. The other study characteristics provided were disease state; whether the study was an FDA-designated orphan study; eligibility criteria (inclusion and exclusion criteria); number of unique treatment procedures and total treatment procedures performed; and visit frequency. Site type and country in which site was conducting the trial were also gathered for each study, along with enrollment and activation timeline data.
Use of recruitment and retention tactics were examined overall and by type of site, global region, and therapeutic area. In addition, the recruitment tactics that most contributed to trial awareness and three most contributing to retention were assessed. A comparison of the most commonly used recruitment and retention tactics was conducted for the 2012 and 2023 studies.
Recruitment tactics were grouped into the following categories: tactics to optimize site databases (including, for example, automated texts and calls and chart pre-screening); tactics for patient outreach (including, for example, websites, social media, brochures, mailings, and other print materials); enhanced referral networks (including, for example, networking, physician referrals, grand rounds, and meetings with clinical colleagues); and diversity and inclusion specific tactics (including, for example, targeted marketing and targeted community outreach).
Site engagement tactics were also collected (including, for example, eNewsletters, mobile apps for data collection, and site webinars). Retention tactics were grouped into three categories, including patient compliance or retention (for example, travel support or reimbursement, patient wearable devices, or patient reminder apps); patient engagement (including, for example, social media, patient communities, or partnerships), and other types of tactics.
Results
Recruitment Tactics
Table 1 represents the ten reported most-used recruitment tactics. The patient recruitment tactics used most often across the sample of trials included brochures and other print materials (56%), websites (44%), and physician referrals (43%). Within approximately 20% of trials, these three most-used tactics were noted as most contributing to overall awareness of the trial.
Although chart pre-screening was reported to be used in 42% of all trials, it was selected most often (32%) as top three in contribution to awareness in the trial. Social media was also a top three contributor to trial awareness among 25% of trials while being used in 37% of trials overall.
Table 1

A list of all tactics and the rate of implementation for recruitment efforts targeted by region is presented in Table 2. In North America (NA), websites (31%), chart pre-screening (28%), and physician referrals (27%) were most common. Similarly, recruitment targeted in Europe, Middle East Africa (EMEA) also most often utilized physician referrals (20%), and chart pre-screening (18%), but brochures/mailings/other print materials (19%) were used more frequently than websites (16%).
The same tactics were used to target Asia Pacific (APAC) at lower rates, brochures/mailings/other print materials were used in 12% of trials and chart pre-screening was used in 10% of trials, while physician referrals were used in 9% of trials. Very few trials used tactics to target Latin America (LATAM), but 6% utilized physician referrals and 5% utilized chart pre-screening.
Table 2

Retention Tactics
Regarding retention tactics, the three most used tactics are also the most often noted as the top three contributing to retention within a trial, as seen in Table 3. These include site training, visit guides, and travel support and reimbursement.
The majority (68%) of trials credited site training to the retention of patients in the trial, and 60% noted travel support or reimbursement as contributing most to retention. Only 5% utilized patient communities, and only 3%–one trial–recognized patient communities as most contributing to retention.
Table 3

Retention tactics by region can be seen in Table 4. Visit guides were used most often for retention efforts in specific regions, except for EMEA, where consent support tools were used in one more trial than visit guides were used in. Site training was commonly used for each region; 27% of trials in North America, 15% of trials in EMEA, 9% of trials in APAC, and 5% of trials in LATAM. Social media was utilized in 24% of trials to retain patients in North America, 13% of trials in EMEA, 2% of trials in APAC, and 1% in LATAM.
Table 4

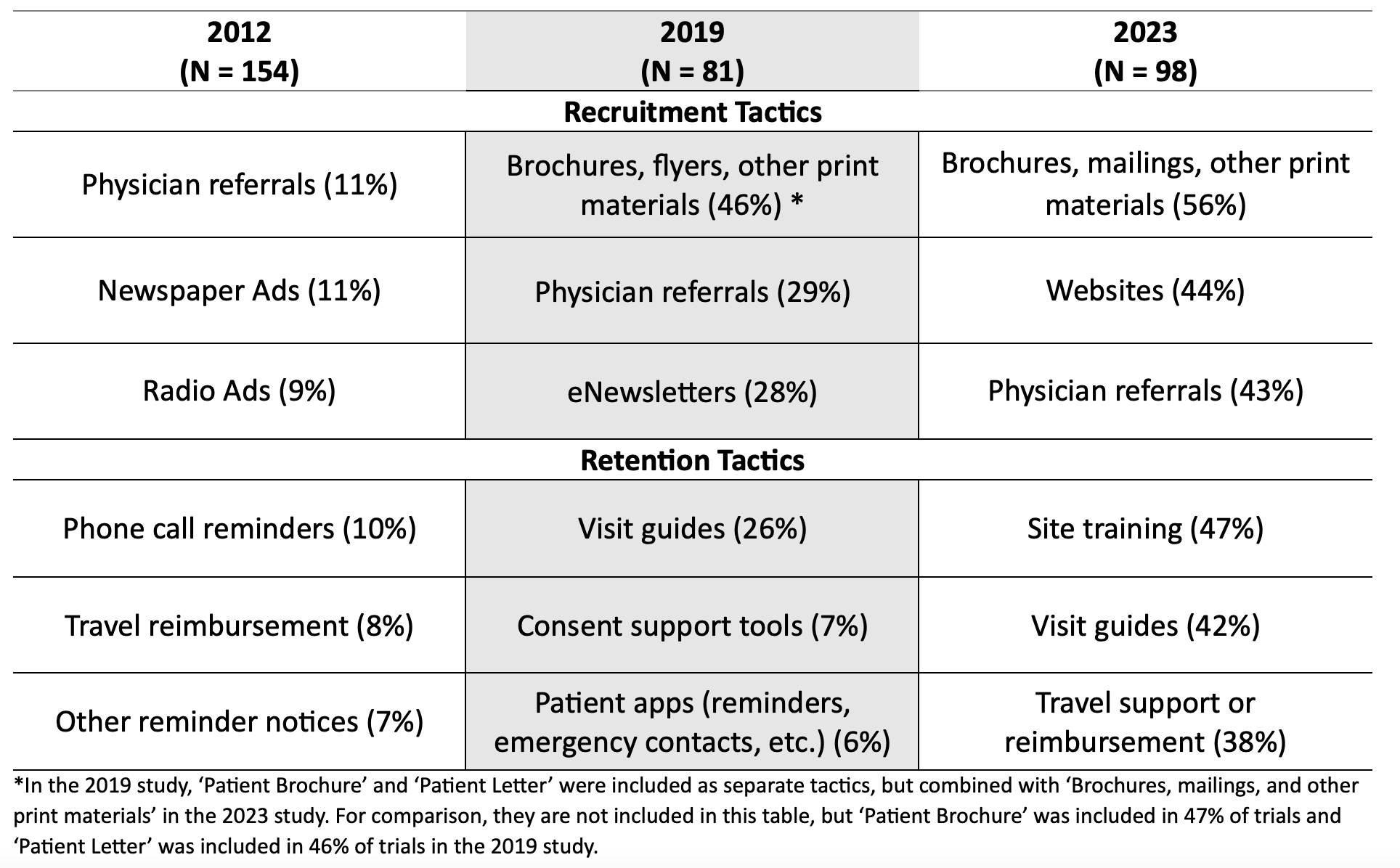
The top three tactics are shown in Table 5. Use of physician referrals was among the most commonly used tactic over time. Although the proportion of studies utilizing physician referrals increased from 11% in 2012 to 43% in 2023, brochures became more frequently used than physician referrals in 2019 and 2023. In 2012, newspaper and radio ads were the second and third most common recruitment tactics, later replaced by eNewsletters in 2019 and websites in 2023.
Websites were used in 3% of studies in 2012, but in 44% of studies in 2023. eNewsletters were not included as a tactic in the 2012 study, but among updated tactics in 2019 in 28% of studies. Only 17% of studies in 2023, however, were utilizing eNewsletters.
Retention tactics varied by year. In 2012, the top three tactics were phone call reminders (10%), travel reimbursement (8%), and other reminder notices (7%). Travel reimbursement was also a top three tactic in 2023, although in 2019 visit guides (26%), consent support tools (7%), and reminder apps (6%) were more prevalent. Site training was utilized as a retention tactic in 7% of studies in 2019 but was the top tactic in 2023 at 47% of studies, along with visit guides (42%) and travel reimbursement (38%).
Table 5. Top Tactics in Each Study
Site Engagement Tactics
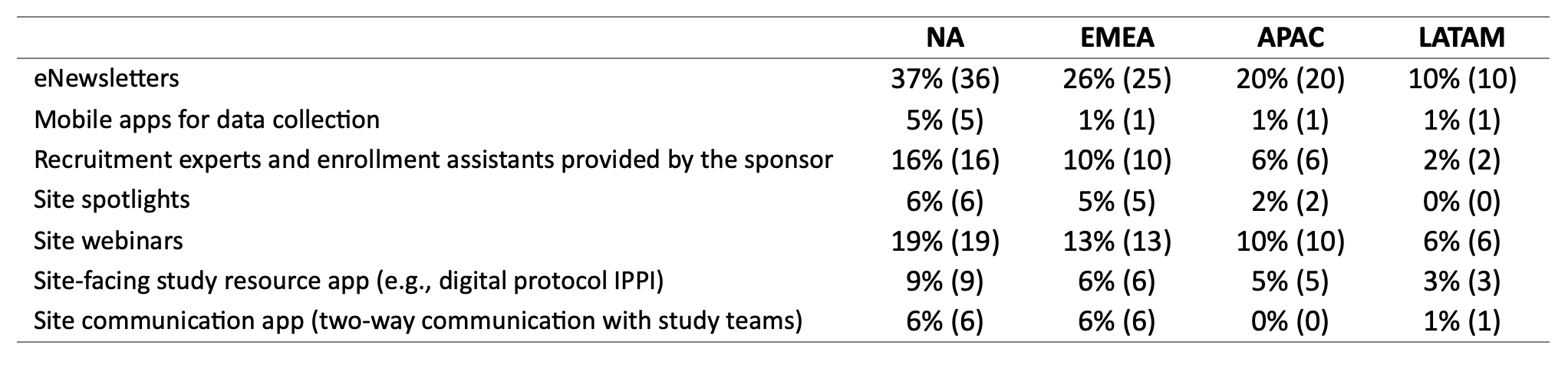
Studies within the sample utilized tactics for site engagement. Most often used site engagement tactics included eNewsletters (37%), site webinars (27%), and recruitment experts provided by the sponsor (19%). The majority (78%) of trials mentioned eNewsletters as a top three tactic contributing to site engagement, and slightly more than half (51%) mentioned site webinars.
Table 6

eNewsletters were used in the most trials to target each region, 37% of trials for North America, 26% for EMEA, 20% for APAC, and 10% for LATAM. About one-fifth of trials utilized site webinars to increase engagement in NA sites.
Site webinars were also used in 13% of trials for EMEA, 10% of trials for APAC, and 3% of trials for LATAM. Smaller percentages utilized mobile apps for data collection and site spotlights to target each region (Table 7).
Table 7. Site Engagement Tactics by Region
Tactic Utilization
Overall, trials used an average of 3.8 recruitment tactics and 2.5 retention tactics. To target North America, trials used 3.5 recruitment tactics and 1.2 retention tactics on average. In Europe on average, trials used 1.3 recruitment tactics and 0.8 retention tactics, while APAC and LATAM used 0.5 and 0.3 recruitment and retention tactics each, respectively. The maximum number of recruitment tactics overall was 14 out of a possible 21 listed or any others mentioned, and the maximum number of retention tactics overall was 10 out of a possible 12 listed or any others mentioned.
Table 8. Patient Recruitment and Retention Tactic Utilization

Regarding tactics aimed to increase engagement with sites, a maximum of 6 site tactics were utilized with an average of 1.3 across all studies. Targeting North American sites, an average of 1 tactic was used with a maximum of 5. Studies utilized an average of 1.1 EMEA targeted tactics, 0.7 APAC targeted tactics, and 0.4 LATAM targeted tactics.
Table 9. Site Engagement Tactic Utilization

Discussion
Recruitment and retention tactics were updated and grouped into different categories in the current study although some comparisons of individual tactics were made from studies conducted in 2012 and 2019. The results of the current study revealed that brochures, including mailings and other print materials, and websites were most commonly used for recruitment. Chart pre-screening was the fourth most frequently cited and identified as most contributing to overall awareness of a trial.
Using an electronic chart review/pre-screening tool, where applicable, can be helpful in identifying potential participants before a study begins, and throughout the enrollment process. Physician referrals were the third most frequently reported tactic and social media was the fifth most used tactic among studies using tactics.
Physician referrals were the tactic most frequently used to recruit patients in EMEA and have been consistently used in the 2012 and 2023 studies. Physician referrals are effective in the EU given universal healthcare and may cast a wider net to patients with certain conditions.
Middle East and North African countries are also thought to be working toward universal healthcare, so these new reforms may open opportunities for researchers to utilize physician referrals for recruitment to clinical research.17 Although still used, chart pre-screening is less common than physician referrals in EMEA and may be a result of consent requirements handling any personally identifiable information as outlined by GDPR (General Data Protection Regulation).18
In Africa, utilizing already existing data from charts may have unique hurdles because of the quality and availability of healthcare data.19 In North America, chart pre-screening was used more often than physician referrals, where HIPAA (Health Insurance Portability and Accountability Act) requirements are less stringent than GDPR.20 Brochures/mailings/other print materials were also used more often than physician referrals and can be cast more widely in North America, where healthcare is not universal.
The top reported tactics were mostly traditional with few mentions of other approaches that were evaluated, such as artificial intelligence for identifying potential patients from medical records, for example, or use of biomarkers, electronic medical records (EMRs), or claims-based matching. As the sample was limited to studies that completed enrollment from 2018 to early 2023, studies that were still enrolling beyond this time that may have included more virtual or innovative tactics were not captured.
In addition, trials being conducted during the pandemic may have implemented online approaches or patient apps, but researchers were not able to capture the data due to the limitations and timing of the data collection. Although traditional tactics were more common, comparisons from 2012 and 2023 indicated increased usage of websites and social media, which may potentially reflect a trend of more online usage post pandemic, especially as a result of increased media coverage during the pandemic on clinical trials.
The 2023 study also included a higher proportion of studies utilizing patient communities and advocacy, reflective of the increased emphasis on patient engagement in clinical trials over the past five to ten years, with patient advocacy groups and communities as critical to facilitating trial participation among diverse patients.21 When comparing retention tactics for these two studies, site training remained foremost, with travel support or reimbursement for 2012 and visit guides for the later study ranked second.
Site training and travel support or reimbursement were assessed as the top two approaches most contributing to retention in the current study. Providing travel support or reimbursement contributes to increasing patient convenience and aligns with current research that reduces patient burden.6 Recent research found that offering support and convenience-enhancing solutions, such as smart phone apps, wearables, eConsent, and concierge services, all correlated with clinical trial satisfaction.6
In terms of strategies that sponsors use to engage sites, eNewsletters and site webinars were used most frequently, with eNewsletters noted as those tactics most contributing to engagement. Site communication apps (two-way communication with study teams), site-facing resource apps were used less but nevertheless rated by companies as contributing to engagement.
Another limitation of the research is that the studies represent a convenience sample determined by companies participating in the study with parameters defined by the researchers and are primarily comprised of large biopharmaceutical companies. Further comparisons are needed based on inclusion of trial data from additional small and mid-sized companies.
The use of recruitment and retention tactics was primarily non-region specific, and some tactics have been identified consistently over time. A more in-depth approach comparing pre- and post- pandemic use of tactics would be beneficial to examine changes at the study and site levels.
Conclusion
The results indicate that use of tactics across studies was mainly comprised of traditional approaches, with some usage of apps for site engagement. Additional research should focus on studies that closed enrollments after the pandemic to examine any changes pre- and post-pandemic or innovative trends in recruitment tactics or variation in enrollment by site type.
About the Authors
Mary Jo Lamberti, PhD, Tufts Center for the Study of Drug Development, Tufts University School of Medicine.
Abigail Dirks, MS, Tufts Center for the Study of Drug Development, Tufts University School of Medicine.
Nicholas Kikuchi, Roche.
Neha Patel Cervantes, GSK.
Ken Getz, MBA, Tufts Center for the Study of Drug Development, Tufts University School of Medicine.
References
1. Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta analysis. BMJ Open 2013;3:e002360 DOI: 10.1136/bmjopen-2012-002360
2. Otobo E, Park C, Rogers J et al.Reinventing inflammatory bowel disease (IBD) clinical trial recruitment using novel digital medicine tools. Journal of Medical Internet Research. 2018; iproc;4(2):e11815.doi: 10.2196/11815
3. Weng, C, Bigger J, Busacca L, et al. Comparing the effectiveness of a clinical registry and a clinical data warehouse for supporting clinical trial recruitment: A case study. 2010. AMIA 2010 Symposium Proceedings. p.867-871.
4. Brogger-Mikkelsen M, Ali Z, et al. Online patient recruitment in clinical trials: Systematic review and meta-analysis. J Med Internet Res. 2020; 22(11):e22179
5. Miyata B, Tafuto B, Jose N. Methods and Perceptions of Success for Patient Recruitment in Decentralized Clinical Studies. Journal of Clinical and Translational Science. 2023; 7:e232: 1-9. doi: 10.1017/cts.2023.643
6. Sine, S., de Bruin, A. & Getz, K. Patient Engagement Initiatives in Clinical Trials: Recent Trends and Implications. Ther Innova Regul Sci 55, 1059–1065 (2021). https://doi.org/10.1007/s43441-021-00306-8
7. Gelinas L, Pierce R, Winkler S, et al. Using social media as a research recruitment tool: Ethical issues and recommendations. Am J Biotech. 2017 Mar; 17(3):3-14. https://doi.org/10.1080/15265161.2016.1276644
8. Reuter K.Social media for clinical trial recruitment: How real is the potential? EMJ Innova. 2020; 4(1): 34-39. https://doi.org/10.33590/emjinnov/19-00121
9. Bender JL, Cyr A, Arbuckle L, et al. Ethics and privacy implications of using the Internet and social medical to recruit participants for health research: A privacy-by-design framework for online recruitment.Journal of Medical Internet Research. 2017; 19(4): e104. https://doi.org/10.2196/jmir.7029
10. Kaitin K. New global recruitment performance benchmarks yield mixed results. Tufts CSDD Impact Report: Analysis and Insight into Critical Drug Development Issues. January/February 2020; 22(1).
11. Lamberti MJ, Smith Z, Henry R, et al. Benchmarking patient recruitment and retention practices.Therapeutic Innovation & Regulatory Science. 2021; 55:19-32. https://doi.org/10.1007/s43441-020-00186-4
12. Lamberti MJ, Mathias A, Myles J, et al. Evaluating the impact of patient recruitment and retention practices. Therapeutic Innovation & Regulatory Science. 2012; 46:573-580. https://doi.org/10.1177/0092861512453040
13. The Future of Clinical Trials is Digital. TLGG Consulting. October 2021 Available at: https://www.tlggconsulting.com/whitepaper/TLGGConsulting-DCT-WhitePaper.pdf
14. Inan OT, Tenaerts P, Prindivile SA. Digitizing clinical trials. npj Digit Med. 2020; 3 (101):1-7. https://doi.org/10.1038/s41746-020-0302-y
15. Harrison F, Coladangelo R, Hardman TC, Marini S. Impact of the COVID-19 Pandemic on Clinical Trial Conduct: Lessons for the Future. Research Gate. 2022; https://doi.org/10.21203/rs.3.rs-1118410/v1
16. Lorenc, A., Rooshenas, L., Conefrey, C. et al. Non-COVID-19 UK clinical trials and the COVID-19 pandemic: impact, challenges and possible solutions. Trials 24, 424 (2023). https://doi.org/10.1186/s13063-023-07414-w
17. Katoue MG, Cerda AA, García LY, Jakovljevic M. Healthcare system development in the Middle East and North Africa region: Challenges, endeavors and prospective opportunities. Front Public Health. 2022 Dec 22;10:1045739. https://doi.org/10.3389/fpubh.2022.1045739
18. Barry V, Farman O. The impact of the GDPR on clinical trial research. October 2018 Available at https://www.nortonrosefulbright.com/en/knowledge/publications/9ba711e7/the-impact-of-the-gdpr-on-clinical-trial-research
19. Musa SM, Haruna UA, Manirambona E, Eshun G, Ahmad DM, Dada DA, Gololo AA, Musa SS, Abdulkadir AK, Lucero-Prisno Iii DE. Paucity of Health Data in Africa: An Obstacle to Digital Health Implementation and Evidence-Based Practice. Public Health Rev. 2023 Aug 29;44:1605821. https://doi.org/10.3389/phrs.2023.1605821
20. National Institute of Health. (2004, February). Clinical Research and the HIPAA Privacy Rule. Retrieved from U.S. Department of Health and Human Services National Institutes of Health: https://privacyruleandresearch.nih.gov/clin_research.asp
21. Alicia Staley, Transforming patient engagement in clinical trials: Moving from a transactional relationship to human-centered care, Drug Discovery Today, Volume 28, Issue 3, 2023, 103509, ISSN 1359-6446, https://doi.org/10.1016/j.drudis.2023.103509. (https://www.sciencedirect.com/science/article/pii/S1359644623000259)
Newsletter
Stay current in clinical research with Applied Clinical Trials, providing expert insights, regulatory updates, and practical strategies for successful clinical trial design and execution.
Improving Relationships and Diversifying the Site Selection Process
April 17th 2025In this episode of the Applied Clinical Trials Podcast, Liz Beatty, co-founder and chief strategy officer, Inato, discusses a number of topics around site engagement including community-based sites, the role of technology in improving site/sponsor relationships, how increased operational costs are impacting the industry, and more.
Reaching Diverse Patient Populations With Personalized Treatment Methods
January 20th 2025Daejin Abidoye, head of solid tumors, oncology development, AbbVie, discusses a number of topics around diversity in clinical research including industry’s greatest challenges in reaching diverse patient populations, personalized treatment methods, recruitment strategies, and more.
Funding Cuts Threaten Diversity in Clinical Research
June 27th 2025In this video interview, Kyle McAllister, co-founder, CEO, Trially, discusses how recent federal funding cuts are likely to undermine research focused on underrepresented populations, and why long-term investment in community-based studies is essential to closing persistent health equity gaps.