Clinical Trial Matching Solutions: Understanding the Landscape
A framework to understand the different approaches of trial matching solutions and the major operational and workflow challenges that all matching solutions share.
Image credit: Egor | stock.adobe.com

The idea of using technology to find patients who meet the eligibility criteria for clinical trials sounds straightforward. A patient or caregiver should be able to find a clinical trial they’re eligible for by entering diagnostic information and medical history and querying a database of available trials. It should also be feasible to enter trial inclusion and exclusion criteria to find all the patients who meet those criteria in a healthcare provider’s electronic health records (EHR).
For three years, I led a cross-industry collaboration to develop an approach for trial matching based on comprehensive genomic profiling for patients with cancer, and I continue to work in the multi-stakeholder trial matching space.
There are many vendors in this landscape, each of which takes a slightly different approach, so it’s important to appreciate the complexity underlying these “simple” challenges. This article provides a framework to understand the different approaches of trial matching solutions and outlines the major operational and workflow challenges that all matching solutions share. It will also help clinical trial stakeholders ask the right questions to identify the options that are most suitable for their needs.
Patient-centric vs trial-centric matching
The first thing to consider is whether the tool is meant to help an individual patient (or group of patients) identify clinical trials they might qualify for.1,2 This is referred to as patient-centric matching and relies on the user to provide the main characteristics of the patient’s diagnostic information, the severity and stage of their disease, and any significant medical history that may disqualify them. This information is used to find trials whose eligibility criteria align with the patient’s condition.
Many solutions offer trial-centric matching in which the eligibility criteria for a single clinical trial or a set of clinical trials are used to query large databases of patient information, such as an EHR, to identify all patients who qualify for any of the trials in the data set.
Who is the intended customer?
The distinction between patient-centric and trial-centric matching leads logically to the first consideration, which is to understand who a matching platform’s intended clients are, literally or figuratively.
Many matching platforms are designed to enable an individual patient, health professional, or caregiver to search for clinical trials they may qualify for. These platforms help to extend recruitment beyond the normal catchment area of participating centers and can empower study participants and deliver motivated potential subjects.
The amount and specificity of information patients have at their disposal may vary, sometimes making it difficult for these tools to identify an exact match to a trial for a targeted therapy. For more common diagnoses and in instances in which many trials are available, such solutions can be quite effective.
Other matching solutions are designed to help clinics, hospitals, academic institutions, or provider networks identify patients who may qualify for trials happening within their organization or catchment area. This can be particularly useful for institutions with a large number of ongoing trials or a large number of patients.
It also eliminates the need for coordinators and investigators to remember the details of individual trials when a suitable patient comes their way. By leveraging the information in their EHRs in this way, organizations have an opportunity to improve their enrollment, making them a more attractive site for additional programs.
One of the limitations of provider-wide matching solutions is that only a subset of identified patients will be available for a specific trial at any given time. Most patients may not be in a position to consider a trial because they are stable on treatment or for other reasons.
Several matching solutions are available to help trial sponsors (i.e., pharma, biotech, or academic sponsors) enroll patients into one or more clinical trials or programs. These services are often one component of a broader range of subject recruitment tactics.
Matching solutions offered to sponsors can include elements of trial-centric or site-based approaches. Some large biopharma companies invite patients to register with them directly for ongoing or upcoming trials.
What information is used to identify matched patients?
It’s important to consider the different types of data that any matching solution needs to query to identify matches. Figure 1 provides a simple schematic to show that all matching solutions must bring together four different types of information to provide actionable matches: patient characteristics, clinical trial eligibility criteria, site identifiers, and site operational status.
Figure. Information types to enable clinical trial matching.
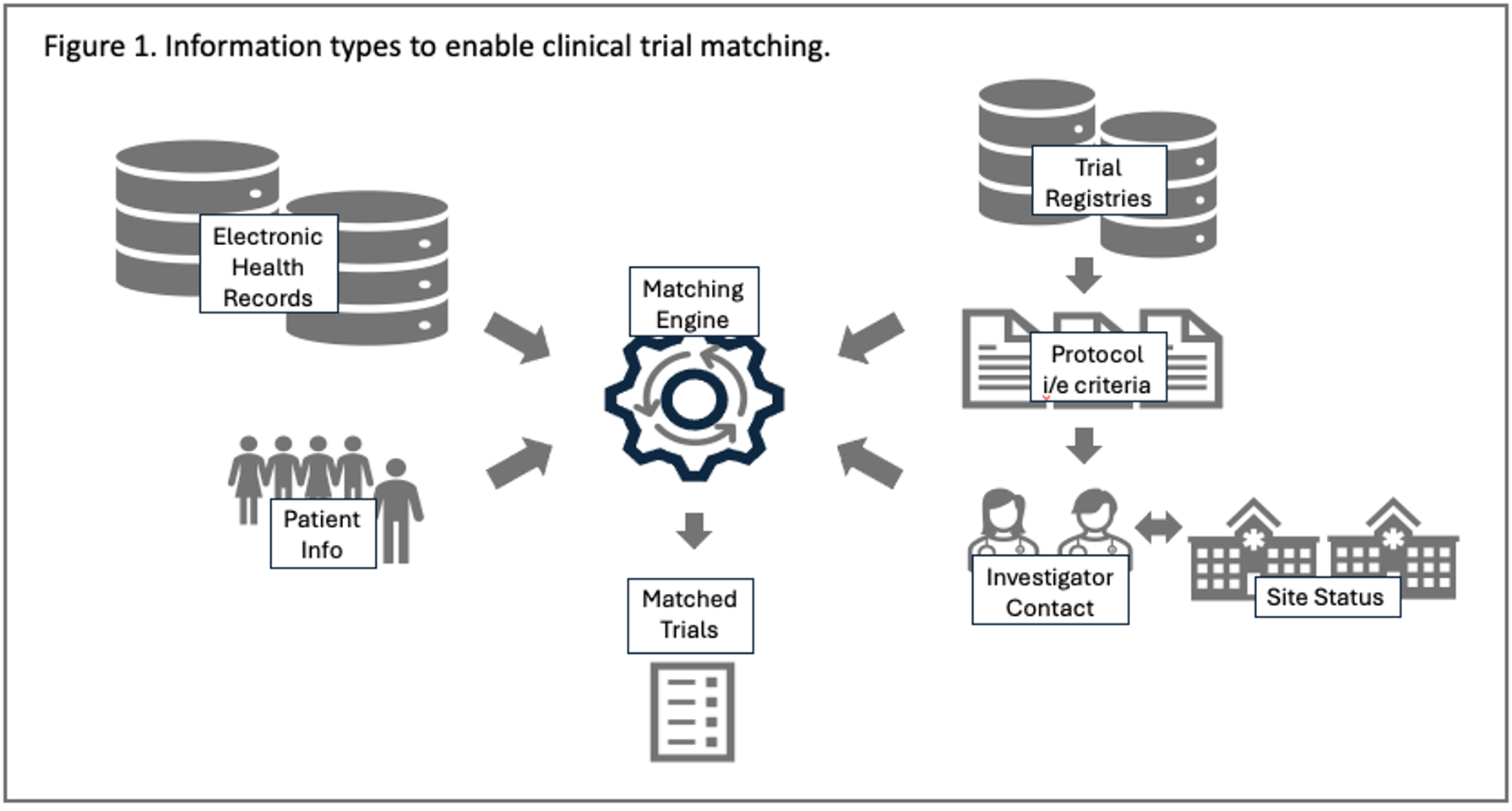
Because each matching transaction relies on different data types and sources, data interoperability and data standards are important considerations.
Patient characteristics meet trial requirements
Of course, the most important information needed to identify potential matches are patient characteristics (disease and diagnostic details, severity or stage, and relevant medical history) and trial eligibility criteria. The source and scope of this information can vary considerably and will have a dramatic effect on the reliability of potential matches the solution can provide.
For patient characteristics, the source of information can be patient-reported, information extracted from the EHR, detailed tumor genetic variations from comprehensive genomic profiling, or a combination of these. It’s important not to underestimate the amount of information that a motivated patient or family member has about their disease. Having access to a more complete medical profile may be critical in assessing a patient’s eligibility for a specific trial.
While it seems obvious that information from the EHR would be a helpful, keep in mind that many clinical concepts will not be explicitly captured in clear verbatim terms, even if aspects of the patient’s condition and the context would make it perfectly clear to a clinician. In addition, records at a specific institution may be quite time-limited, with many relevant aspects of a patient’s history captured in their primary care or other medical records.
There are two considerations regarding trial eligibility criteria. First is the number of eligibility criteria considered for a particular trial, and how they are curated and configured in the matching solution. Inclusion and exclusion criteria can have complex wording and contain multiple concepts joined by logical connectors.
The following is a representative example of the disease description from a clinical trial listing on clinicaltrials.gov. This is for a Phase II study in first-line treatment of advanced non-small cell lung cancer (NSCLC) [NCT05557591. Cited February 5, 2024]:
Participants with non-squamous or squamous histology NSCLC with stage IIIB or stage IIIC disease who are not candidates for surgical resection or definitive chemoradiation per investigator assessment or stage IV (metastatic) disease who received no prior systemic treatment for recurrent or metastatic NSCLC.
Note that this description includes several discreet concepts (e.g., disease, histology, stage, excluded prior treatments and treatment options) that would need to be verified against the available patient information. Interventional studies often have dozens of eligibility criteria. While it is possible to identify reasonable matches with a smaller selection of key criteria, it’s important to understand how these are prioritized within the matching solution.
A major consideration is the set of trials being considered within the platform. Solutions providing trial-centric matching may be based on, or prioritize, a single study or a very small number of trials. For solutions matching patients across a health provider organization, the set would be all trials going on at that center. And some solutions also look for matches against all trials found in a comprehensive registry, such as clinicaltrials.gov, or the EudraCT database.
Site and investigator information
A potential match identified by the solution should include actionable contact information that can be used to follow up. This can be a serious limitation where trials are extracted from clinicaltrials.gov since many listings provide incomplete site details. It’s important to understand the quality of the contact information, and the site’s capacity to respond to queries regarding potential matches.
Site status and operational information
Connecting an eligible patient to a trial and an identifiable site is insufficient if the study is no longer recruiting at that location. Complete trial information should include the status of participating sites in real time, at a minimum the start and end of enrolment. As the industry implements more cohort-driven trial designs this information will be increasingly critical.
How does the matching algorithm work and how actionable are the results?
A matching solution can be considered a special type of Clinical Decision Support System (CDSS). Like other CDSS,3,4 matching platforms involve a communication interface, a mechanism to query a knowledge base (i.e., of clinical trials and patient information) and return a curated recommendation. While most matching platforms use proprietary algorithms to calculate their recommendations, it’s important to understand which types of information are considered in identifying matches. It is also interesting to note the emergence of open-source matching solutions on platforms such as GitHub.
Another important aspect to consider is the reliability of matches from the system. Potential matches will normally require additional information and adjudication before they can be acted upon. That is not necessarily a problem, but clients of these systems should have a good understanding of expected results and a plan for the resources to complete this work. In fact, this type of resource is an important part of the service offering (and pricing model) of many matching providers.
The role of artificial intelligence (AI), natural language processing (NLP) and large language models (LLMs)
There are many places in this process where AI, NLP, or LLMs would be very useful. This could include using NLP for “entity extraction” from trial eligibility criteria or patient records, leveraging LLMs to detect patterns from unstructured clinical notes in the EHR, or detailed genetic testing, in which a diagnosis may not be stated verbatim. A full exploration of this is out of scope for this article but will be explored in a future publication.
Everything comes down to “the last mile”
Once a matching platform identifies good matches between trials and potential patients and provide detailed enough information to take the next step, what happens next? Understanding the next steps and making sure the right resources are available to execute them is a critical challenge for all matching solutions.
Typically, once a potential match is identified, aspects of the patient’s eligibility would be further investigated by verifying information in their health record. If all checks out, then the site and the patient would contact one another to discuss other considerations about trial participation. Administrative details such as medical referrals, insurance concerns, and the logistics of trial participation would be discussed with the patient.
If those details are amenable to both sides, and the patient is in geographic reach of the site, only then would the patient consider participation and proceed to the full informed consent process. These last steps constitute a real workflow challenge for sites and can’t be underestimated.
Key questions to guide the choice of a clinical trial matching solution
By way of summary, there are several things to consider when evaluating the right approach to clinical trial matching. The following questions will serve as a simple outline:
- Who is the client that the matching solution is intended to serve? Will it help individual patients or help a healthcare provider institution or sponsor to improve enrollment across their organization?
- What sets of information will the solution leverage to identify matches, and how will the solution receive that information? Does the solution consider a single trial, a select portfolio of trials, or all trials in a public registry?
- Will patients be matched to trials one at a time or will the system match across larger groups of patients?
- How will patients contact participating sites (or vice versa)? How will the solution or workflow ensure that patients and sites are within geographic reach of one another?
- How will potential matches be verified, and what steps are needed before a potential study participant would start the informed consent process? Who will be responsible to complete those steps?
Clinical trial matching is an idea whose time has arrived. This review will assist stakeholders and decision-makers create a plan to maximize the value of these solutions for sponsors, investigator sites, and especially for patients.
About the Author
Todd Georgieff has worked in drug development for over thirty years. From 2020 until early 2023, Todd led the Research and Development (trial matching) workstream for the Precision Cancer Consortium, a cross-pharma collaboration to improve global patient access to comprehensive genomic testing in routine care and clinical trials. He has extensive experience in Clinical Operations, Therapeutic Area Leadership, and the digitalization of clinical research. He is currently providing strategic consulting services to sponsors, industry consortiums, software companies, and venture capital firms.
References
- Klein H et al. Matchminer: an open-source platform for cancer precision medicine. NPJ Precis Oncol. 2022 Oct 6;6(1):69. doi: 10.1038/s41698-022-00312-5.
- Kaskovich S et al. Automated Matching of Patients to Clinical Trials: A Patient-Centric Natural Language Processing Approach for Pediatric Leukemia. JCO Clin Cancer Inform. 2023 Jul;7:e2300009. doi: 10.1200/CCI.23.00009. PMID: 37428994.Top of FormBottom of Form
- Haddad T et al. Accuracy of an Artificial Intelligence System for Cancer Clinical Trial Eligibility Screening: Retrospective Pilot Study. JMIR Med Inform 2021;9(3):e27767
- Sutton RT et al. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digital Medicine (2020) 3:17 ; https://doi.org/10.1038/s41746-020-0221-y
Newsletter
Stay current in clinical research with Applied Clinical Trials, providing expert insights, regulatory updates, and practical strategies for successful clinical trial design and execution.
Improving Relationships and Diversifying the Site Selection Process
April 17th 2025In this episode of the Applied Clinical Trials Podcast, Liz Beatty, co-founder and chief strategy officer, Inato, discusses a number of topics around site engagement including community-based sites, the role of technology in improving site/sponsor relationships, how increased operational costs are impacting the industry, and more.
Reaching Diverse Patient Populations With Personalized Treatment Methods
January 20th 2025Daejin Abidoye, head of solid tumors, oncology development, AbbVie, discusses a number of topics around diversity in clinical research including industry’s greatest challenges in reaching diverse patient populations, personalized treatment methods, recruitment strategies, and more.
Funding Cuts Threaten Diversity in Clinical Research
June 27th 2025In this video interview, Kyle McAllister, co-founder, CEO, Trially, discusses how recent federal funding cuts are likely to undermine research focused on underrepresented populations, and why long-term investment in community-based studies is essential to closing persistent health equity gaps.