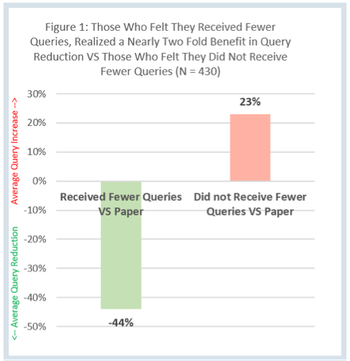
The Site Impact Survey was designed by Clinical Ink to evaluate clinical research coordinator and investigator experiences with Clinical Ink’s eSource system compared to paper source.


The Site Impact Survey was designed by Clinical Ink to evaluate clinical research coordinator and investigator experiences with Clinical Ink’s eSource system compared to paper source.

In our latest webcast that aired live October 14, Oracle Health Sciences brought together three presenters from smaller or medium-size organizations in an effort to educate similar-sized companies on the benefits of EDC.

Even with the rapid increase in clinical trials and demand for cloud-based systems, doing business in China is not easy if you are unfamiliar with the territory.

Survey provides new benchmarks on e-solutions adoption, but actual impact data remains elusive.

How to satisfy regulatory concerns about EDC data integrity and site controls over its source records.

In clinical trials, the ability to effectively plan, collect and maintain essential clinical trial documentation is challenging.

Last month, Veeva Systems unveiled the results of its 2015 Paperless TMF Survey: Annual Report. The survey is in its second year, and as Jennifer Goldsmith, vice president of Veeva Vault, told Applied Clinical Trials, it was interesting and significant to report on the year-over-year results.

Understanding the roles of eTMF, CTMS, and study startup solutions in the clinical trials process

eSource is not a new term. But eSource does seem to be getting a lot of attention lately.

Historically, some longstanding challenges have hindered efforts to integrate patient data from EMRs (Electronic Medical Record systems) with EDC (Electronic Data Capture) systems.

ERT’s announcement of its intention to acquire PHT makes more than sense. And when speaking with the companies’ CEOs, Jim Corrigan and Philip Lee respectively, the potential for success is greater than any challenges the current clinical trials market could throw their way.

Combing for clinical trials patients and the best way to make the match between trial and patient is a multifaceted task.
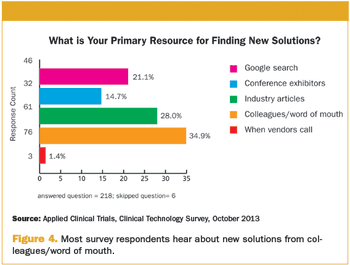
As we move towards cloud-based technologies, lingering questions and concerns remain.
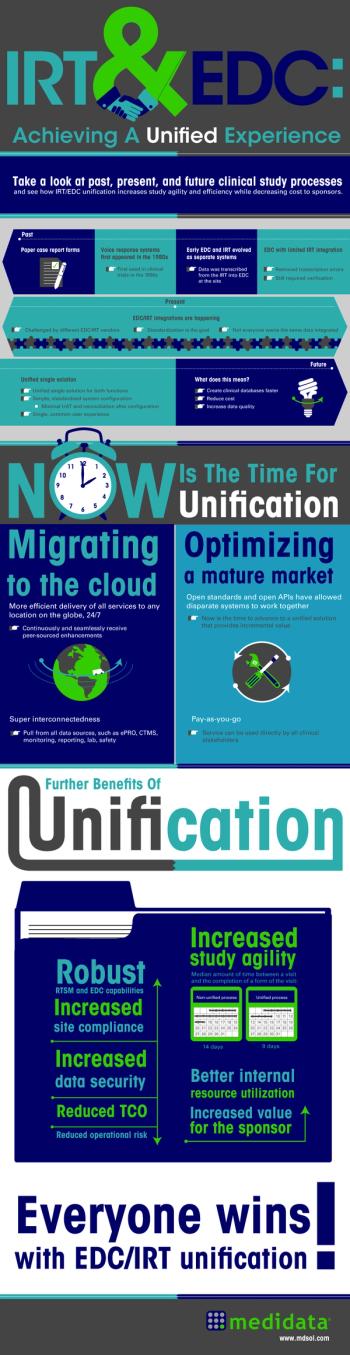
Take a look at past, present, and future clinical study processes and see how IRT/EDC unification increases study agility and efficiency while decreasing cost to sponsors.
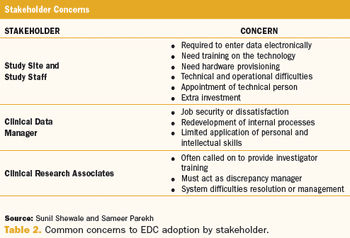
Does the emerging world still lag behind in EDC adoption?


Overcoming clinical research inefficiencies to improve trial outcomes.

Efficiency and ease of use-bringing technologies together into a converged suite.

Using interactive response technology to avoid unblinding in randomized controlled trials.
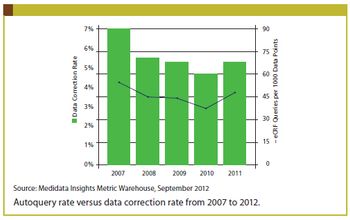
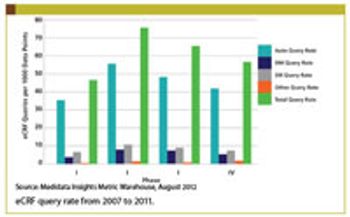


Clinical professionals can recreate CTMS by applying modern technology in a new way.

Obsession with shorter cycle times has been mistakenly drilled into all aspects of development.
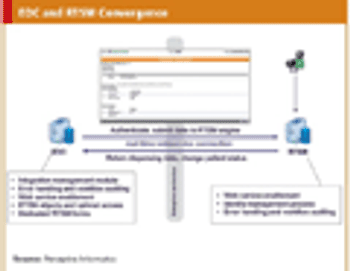
Examination of eClinical shows that it is more than just bold claims, there is substance.