
These three areas will be at center of drug development discussions in the year ahead.

These three areas will be at center of drug development discussions in the year ahead.

Robert Califf addresses questions about drug pricing at the Senate hearing to weigh his appointment to be the next commissioner of FDA.

Pharma and biotech companies are working with academics and health care organizations to establish systems for collecting and sharing the results of clinical trials, but they have far to go, according to a recent analysis of industry adherence to data transparency requirements. A report from the non-profit Bioethics International finds that for a group of new drugs approved by FDA in 2012, large pharma companies fell “below legal and ethical standards” for making public information from the relevant clinical trials.
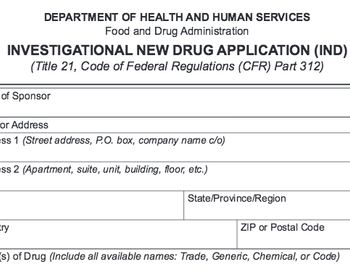

Tips for avoiding a clinical hold.

The White House nominates Robert Califf to head FDA, but will he be confirmed?

Approved by the European Parliament in April 2014 is EU Regulation 536/2014, which replaces the decade-old Directive 2001/20/EC – the Clinical Trials Directive. While approved last year, it does not apply earlier than May 28, 2016-leaving interested parties only nine months to prepare for its arrival.

FDA needs to expand and empower its Office of Health & Constituent Affairs (OHCA) and its Patient Liaison Program to better coordinate interactions with individuals and physicians seeking expedited access to experimental therapies

The schedule is particularly tight this time because the user fee reauthorization process coincides directly with the presidential elections.

The era of personalized medicine is fast approaching and, in many therapeutic areas such as oncology, is already underway.
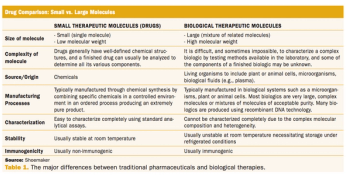
A regulatory perspective on the current state of protein science and the implications for biosimilar approval.

Current clinical trials are regarded as “too slow, too expensive, not reliable, and not designed to answer the important questions,” according to FDA’s new deputy commissioner for medical products &tobacco, Robert Califf.

Among the multiple proposals for stimulating drug development and revising regulatory processes, the 21st Century Cures initiative includes several provisions designed to streamline clinical research and the amount of data required to gain FDA approval of certain indications.

Demands from patient advocacy groups for broader subgroup representation in clinical trials has generated a new drug trial transparency initiative at the Center for Drug Evaluation and Research (CDER).


Media rebuke of Europe’s Innovative Medicines Initiative thrusts debate into the public arena.

The productivity of the U.S. biomedical research enterprise is undergoing a broad re-examination.

FDA commissioner Margaret Hamburg has received praise and plaudits as she exits the Food and Drug Administration this week after six years on the job.
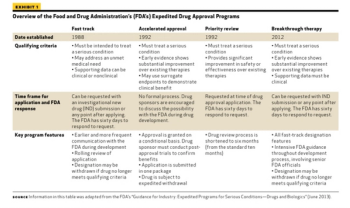
The FDA works to protect public health by balancing the requirements for extensive safety and efficacy data prior to approval, and the need to expeditiously issue approval decisions to ensure medicines that could save or dramatically improve patients’ lives are available as soon as possible.

Vice President of Global Regulatory Affairs, Theorem Clinical ResearchIntroduction

While such discussions have advanced in Europe, two key omissions from the dialogue may limit any real changes.

The main surprise at the Jan. 7, 2015 meeting of FDA’s Oncologic Drugs Advisory Committee was the panel’s strong support for a drug developed under a very different model from most cancer therapies.

FDA set several milestones in approving more new, important drugs and biologics in 2014.

In a health policy world where competition for profile is tough, a vacuum at the top is a serious disadvantage.