
Examining the two areas of weakness cited in FDA draft guidance.

Examining the two areas of weakness cited in FDA draft guidance.

Increased use of remote assets will showcase new endpoints derived from digital health technologies.

Outlining the potential of three mHealth technology approaches in enabling novel and more robust clinical outcomes measurements.

There have been a number of significant scientific and regulatory milestones driving the adoption of electronic patient-reported outcomes in clinical trials since the first screen-based ePRO solution, Minidoc, appeared in 1980.

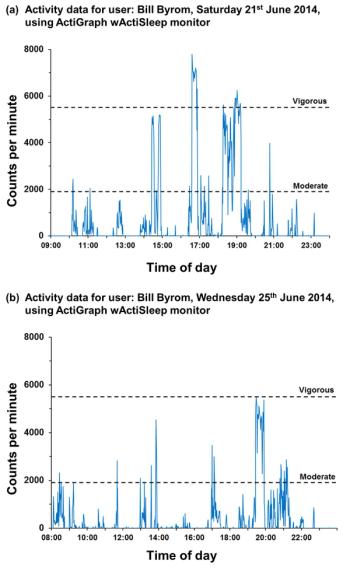
Researchers are now looking to design their trials around a bring-your-own-device (BYOD) strategy, which allows participants in a clinical trial to provide study data using their own internet-enabled hardware.
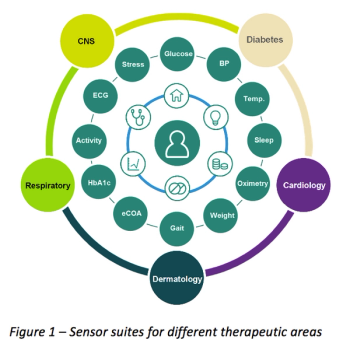
Investment in mHealth and the adoption of wearables are significant initiatives that are changing the clinical trial process toward a more patient centered approach. These wearable technology initiatives have the potential to be the most innovative advances in drug development.
Survey uncovers the challenges, myths, and potential useful strategies associated with BYOD adoption.

Miniaturization of sensors and circuitry has enabled huge proliferation in the development and commercialization of wearable and external monitoring devices for health and wellness.

Efficiency and ease of use-bringing technologies together into a converged suite.
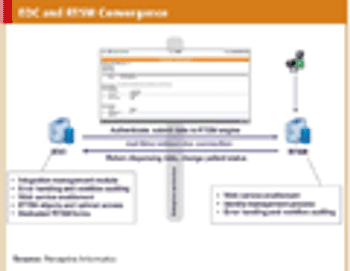
Examination of eClinical shows that it is more than just bold claims, there is substance.
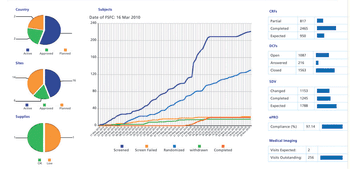
Building a technology foundation to support clinical development efficiency.

A list of the most influential events affecting the clinical trials industry over the last 30 years.
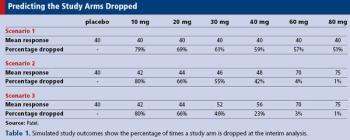
How simulation can help in the planning and implementation of adaptive clinical trials.
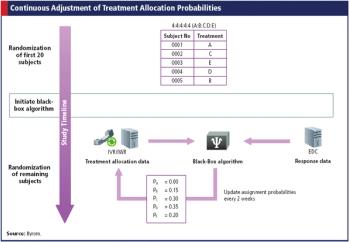
With help from technology, adaptive trials can enhance dose selection and reduce time between phases.
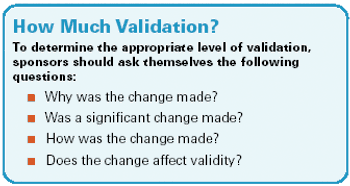
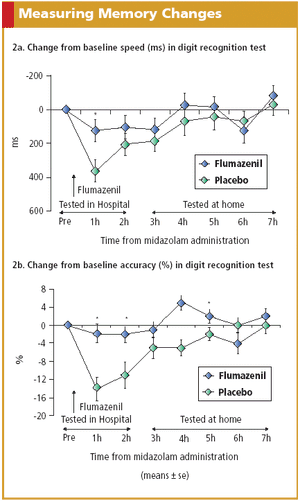
The 2006 Guidance reveals the advantages of using electronic modalities, such as IVR, when collecting patient-reported data.
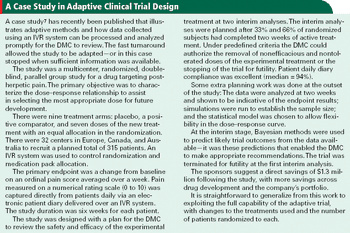
Interactive voice response systems are a good match for adaptive clinical trials and can help keep investigators in the dark.

Interactive voice response systems are a good match for adaptive clinical trials and can help keep investigators in the dark.
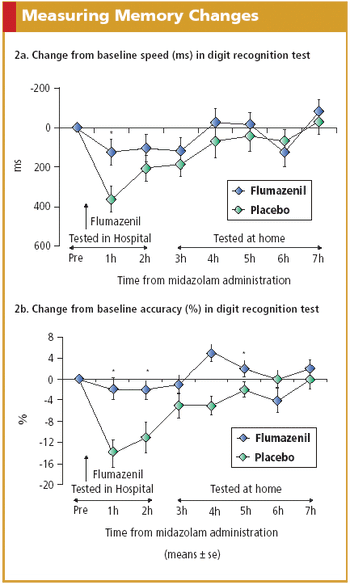
Electronic solutions such as IVR systems, PDAs, and digital pens exhibit advantages over paper and pencil in PRO data collection.

Electronic solutions such as IVR systems, PDAs, and digital pens exhibit advantages over paper and pencil in PRO data collection.

Published: May 16th 2023 | Updated:

Published: March 1st 2013 | Updated:
Published: November 1st 2011 | Updated:

Published: September 1st 2010 | Updated:
Published: February 1st 2011 | Updated:
Published: June 1st 2006 | Updated: