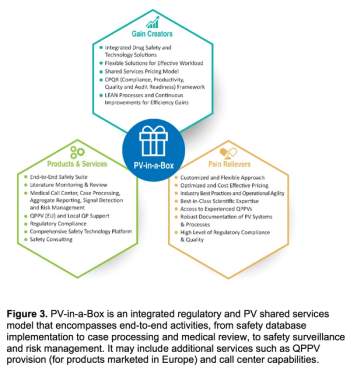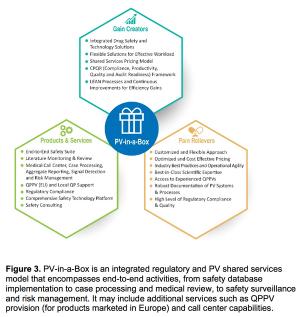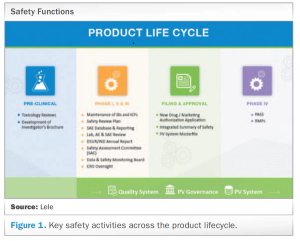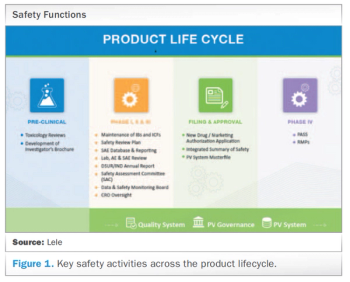
Meeting today’s complex regulatory demands when it comes to drug safety and pharmacovigilance can be especially challenging for small and medium-sized organizations. This report presents the benefits for these companies in outsourcing such activities to functional service providers (FSPs) during clinical trials and post-approval.