
The importance of examining this generation’s influence on the clinical trial value chain.

The importance of examining this generation’s influence on the clinical trial value chain.
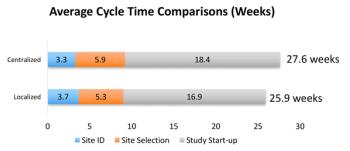
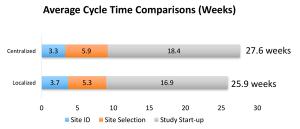
Numerous factors can adversely impact study startup and its efficiency, in an industry plagued by rising development costs and increasing complexities.

As the size, complexity, duration, cost, and globalization of clinical trials has grown, pharmaceutical and biotech companies have moved to outsource clinical activities to CROs to achieve a wide range of objectives.

With the availability of workflow-based study start-up tools, proactive planning is within reach for stakeholders who view this function as pivotal to improving clinical trial quality.

Pharma has been transitioning from its paper based methods of the past toward automated cloud based systems in order to offset the cost of drug development. The emphasis is now shifting toward improved study startup processes for shorter clinical trial timelines.
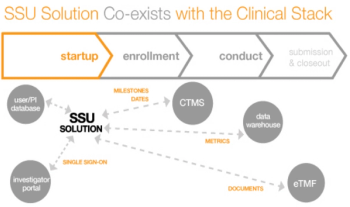
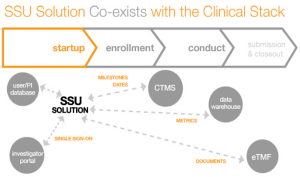
The need for more efficient clinical trials is driving greater use of cloud-based solutions, especially with the rise in globalization
Published: July 15th 2015 | Updated:

Published: September 16th 2016 | Updated:

Published: May 18th 2017 | Updated:

Published: October 30th 2017 | Updated:
Published: February 15th 2018 | Updated:

Published: March 1st 2018 | Updated: