
How optimizing RBQM risk detection reduces the efforts caused by false signals.
Jonathan Rowe, PhD, MS, MA, has worked in the pharmaceutical industry across clinical, quality and corporate teams for over 25 years. He currently leads ZS’s clinical development quality, operations and risk management functions from the New York office.

How optimizing RBQM risk detection reduces the efforts caused by false signals.

Identifying and providing clarity on the GCP quality and risk concerns associated with DCT modalities.

Optimizing feasibility through increased data collection.
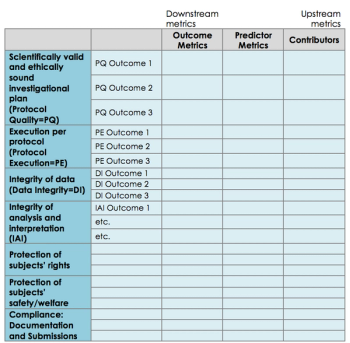
A case study by Pfizer & The Avoca Group on the development of an overarching strategy for Pfizer’s clinical trial quality metrics.

Published: September 7th 2022 | Updated:
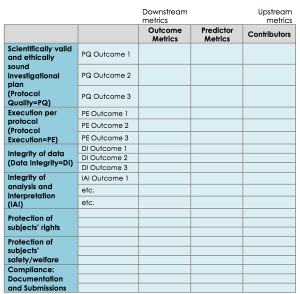
Published: July 8th 2015 | Updated:

Published: December 16th 2021 | Updated: