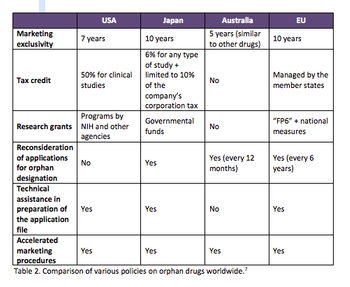
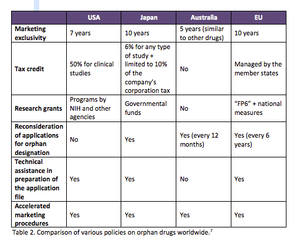
Orphan medicines have become a formidable R&D segment with robust growth that is expected to continue. Expansion is needed to cope with the issues of development and patient recruitment. An understanding of regulations, therefore, is critical.
Published: April 12th 2016 | Updated: