
Differing levels of trust in clinical trials information channels across diverse populations is examined in this research.

Differing levels of trust in clinical trials information channels across diverse populations is examined in this research.

Clinical trials must rely on internet surveys to incorporate the needs of industry and patients, but how can results be improved?

Survey among readers evaluates the trajectory of RWD and how it can be more widely adopted.

Survey taken by pharma and CRO executives evaluates how effectively various programs convert RWD into usable data.

Survey taken by pharma and CRO executives evaluates attributes of large datasets.

Identifying careless responding to improve survey construction and results.

Survey among pharma and CRO executives evaluates current usage of RWD.

A model to identify the services and resources sites need to conduct high-quality clinical trials.
Most people in our poll said it was a service, but others disagreed. Find out more in this follow-up from Michael Howley PA-C, PhD and Associate Clinical Professor, Department of Marketing at the LeBow School of Business at Drexel University.

This three-step process can help managers accurately assess how patient-centric their trials really are.

Adopting scientific quality measurement that recognizes that clinical trials are a service will reap benefits.

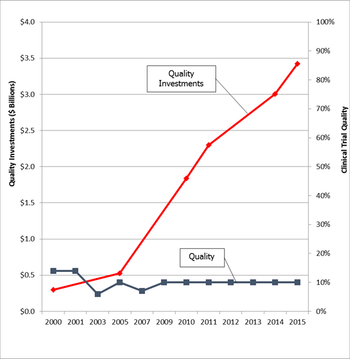
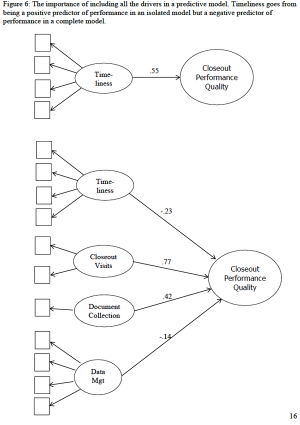
Considerable quality investments over the past decade have had little demonstrable impact on clinical trial performance and quality.

In early January, Novartis selected Qualcomm Life as a global digital health collaborator for its Trials of The Future program.
This article is the first in a series of articles that will review the development and findings of the first scientific measurement of quality in clinical trials.
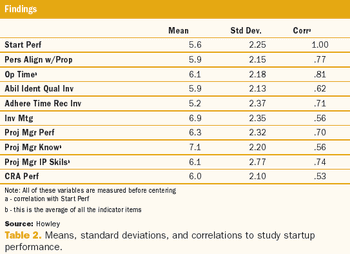
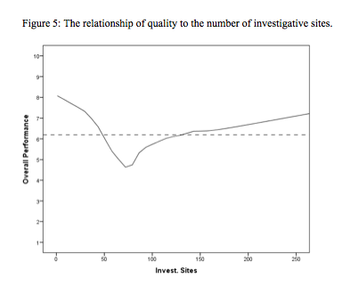
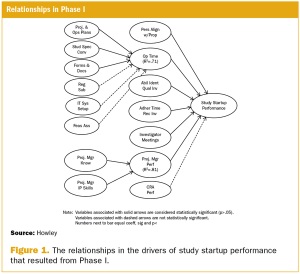
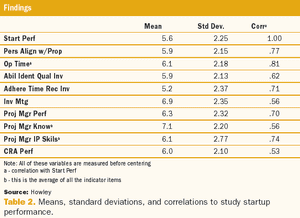
A two-phase statistical analysis identifies the key performance drivers in clinical trial startup.

Effective clinical trial management depends on accurate and unbiased performance measurement.
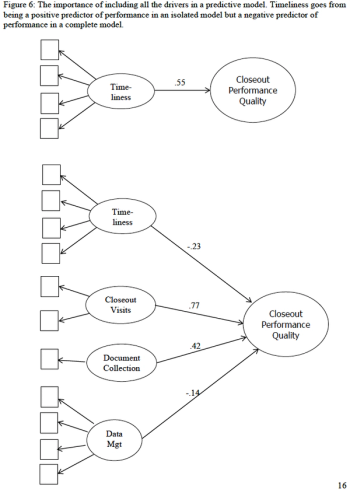
Organizations involved in clinical trials have embraced the idea that they should collect performance measures to assess how well they conduct clinical trials.
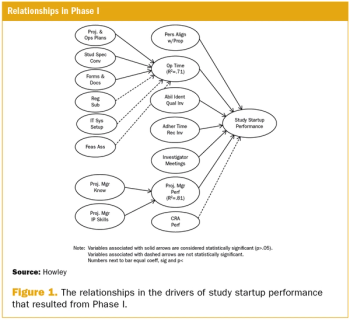
Study startup underperformance and inefficiency has been a problem for executives who manage clinical research trials for decades.

Published: September 22nd 2022 | Updated:
Published: February 27th 2014 | Updated:
Published: February 20th 2014 | Updated:

Published: May 27th 2014 | Updated:
Published: June 1st 2014 | Updated:
Published: January 13th 2015 | Updated: