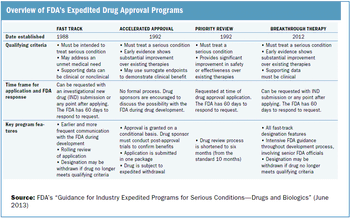
Outlining the requirements and benefits of FDA's four expedited drug approval pathways.

Outlining the requirements and benefits of FDA's four expedited drug approval pathways.
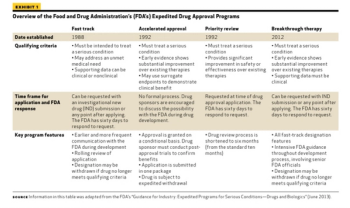
The FDA works to protect public health by balancing the requirements for extensive safety and efficacy data prior to approval, and the need to expeditiously issue approval decisions to ensure medicines that could save or dramatically improve patients’ lives are available as soon as possible.
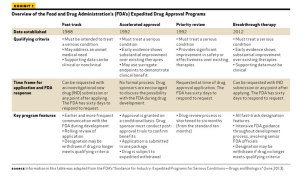
Published: March 11th 2015 | Updated:
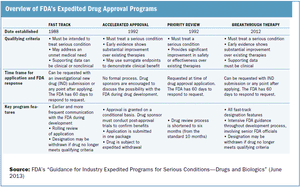
Published: April 1st 2015 | Updated: