
Spotlight on clinical trials created by COVID-19 pandemic has forced regulatory officials around the world to expand disclosure requirements on results.

Spotlight on clinical trials created by COVID-19 pandemic has forced regulatory officials around the world to expand disclosure requirements on results.
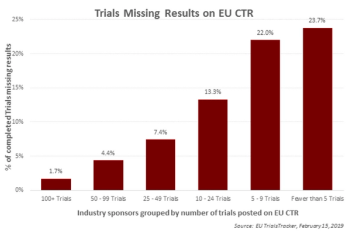
Transparency in clinical trials doesn't have to be difficult but requires attention writes Thomas Wicks, Chief Strategy Officer for TrialScope. Smaller organizations tend to lag in their commitments to clinical data sharing and be non-compliant with regulations, but the trend is shifting.


To manage the growing scope and complexity of global disclosure regulations and trial transparency initiatives, companies are advised to create a cross-functional clinical transparency committee.

The FDA Amendment Act is on its way toward implementation at years’ end. We reveal the facts on clinical trial disclosure for compliance with these new requirements.

Published: December 12th 2016 | Updated:

Published: June 6th 2018 | Updated:

Published: January 18th 2019 | Updated:
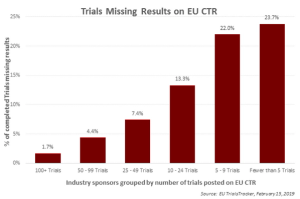
Published: April 26th 2019 | Updated: