
With significant technology advances in ECG recording through the years, the need to expand on traditional monitoring baselines-and include new variables such as time when designing clinical trial protocols-is important.

With significant technology advances in ECG recording through the years, the need to expand on traditional monitoring baselines-and include new variables such as time when designing clinical trial protocols-is important.
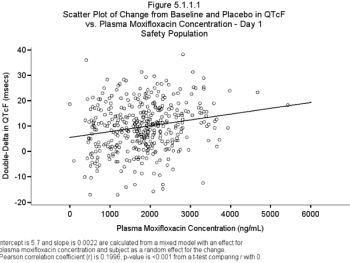
The US FDA, in open meetings, stated the Thorough QT trial "must" be replaced.

The sponsor of a recent Phase II global study, conducted at more than 50 sites, invested in a centralized digital pathology core lab to ensure a homogenous and representative study population.


The responsibilities regarding CV safety for drug developers are constantly evolving.
Centralized approach for Phase III studies enhances the quality and integrity of collected data.

Published: January 10th 2013 | Updated:

Published: February 1st 2013 | Updated:

Published: January 1st 2012 | Updated:
Published: November 1st 2010 | Updated:
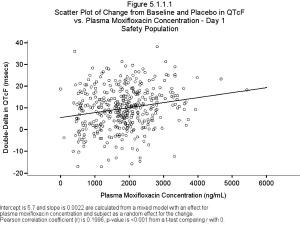
Published: September 22nd 2014 | Updated:

Published: February 22nd 2016 | Updated: