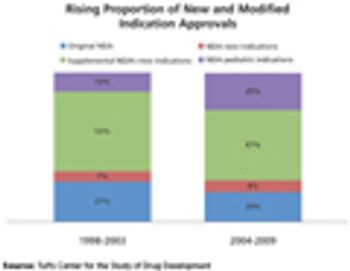
Tufts Center for Drug Development

Tufts Center for Drug Development
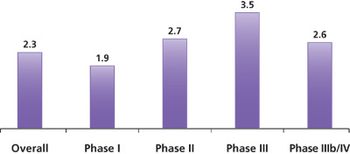
A recent study conducted by the Tufts Center for the Study of Drug Development (Tufts CSDD) found that more than half of all protocols require one or more amendments.
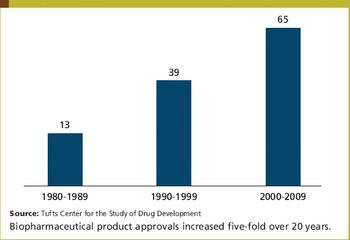
Biotechnology is delivering substantially higher numbers of new product approvals. During the period 2000 to 2009, 65 biopharmaceutical products received US marketing approval, nearly double the number of products approved during the 1990 to 1999 period and five times the 1980 to 1989 level.
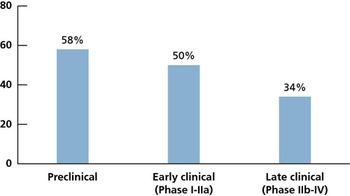
A 2010 survey conducted by the Tufts Center for the Study of Drug Development (Tufts CSDD) finds that sponsor companies are allocating resources, modifying organizational structures, and increasing investment in the development of personalized medicines (i.e., tailoring of medical treatment and healthcare delivery based on individual patient characteristics including genetic, molecular, and imaging).
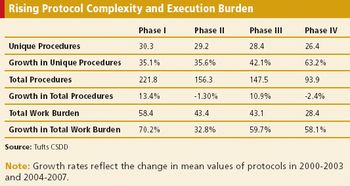
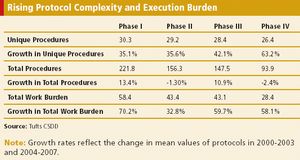
Protocol complexity, as measured by the number of unique procedures and their frequency and the number of subject eligibility criteria, has risen dramatically in comparisons of protocol characteristics between two time periods, 2000-2003 and 2004-2007.
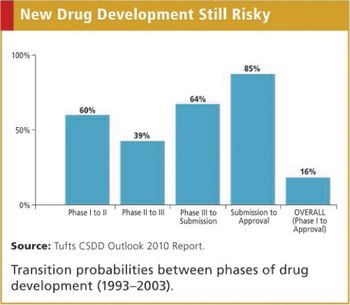
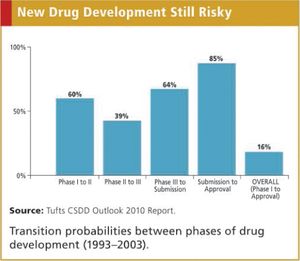
It now costs more than $1 billion and takes more than seven years, on average, to conduct clinical trials and win regulatory approval to market a new drug. Not only are development costs high and rising steadily, but also only one out of every six self-originated drugs developed successfully completes clinical testing and obtains marketing approval.
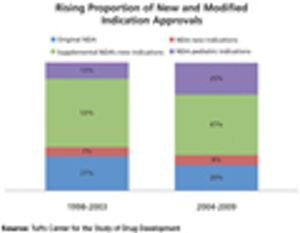
Published: January 1st 2012 | Updated:
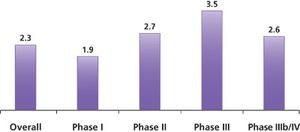
Published: September 1st 2011 | Updated:

Published: June 1st 2011 | Updated:

Published: March 1st 2011 | Updated:
Published: November 1st 2010 | Updated:
Published: May 1st 2010 | Updated: