
Undertaking an assessment of the POS can be coupled with the product profile and safety assessments of compounds prior to launching a clinical trial.

Undertaking an assessment of the POS can be coupled with the product profile and safety assessments of compounds prior to launching a clinical trial.

An overview of all the drug approvals that were secured in 2017.

The recent landmark international trial that was conducted at 23 cancer centers around the world including Memorial Sloan Kettering Cancer Center, New York, had results published last month.

One of the primal questions clinical trialists engaged in clinical research and development always face is do we “keep or kill” a drug based on the data in hand.

There are various estimates in the public domain that applying big data strategies could potentially generate up to $100 billion in value annually across the US healthcare systems.
As I was trying out a series of corrective lenses at an optometrist last week to choose the one that finally provided me the clarity that I was looking for, it made me pose a profound question to myself about perception and reality.

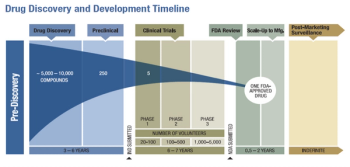
The overall burden of drug development has increased exponentially over the last decade or so.

Developing and utilizing a comprehensive feasibility strategy to avoid risk and ensure the most efficient clinical trial.

Published: April 9th 2014 | Updated:

Published: April 1st 2009 | Updated:
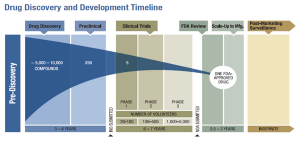
Published: May 8th 2014 | Updated:

Published: September 18th 2014 | Updated:

Published: February 23rd 2015 | Updated:

Published: September 21st 2015 | Updated: