
Applied Clinical Trials
Payers seek more comparative drug data from sponsors and independent researchers.

Applied Clinical Trials
Payers seek more comparative drug data from sponsors and independent researchers.

Applied Clinical Trials
European Medicines Agency adopts new approach to drug development process.

Applied Clinical Trials
This new course aims to provide clinical research physicians with good clinical practice knowledge.

Applied Clinical Trials
Teaming up with a staffing partner can help companies find the right CRA for their trial.

Applied Clinical Trials
Open-label extension (OLE) studies are common, but they do not receive as much attention as traditional Phase I through Phase IV studies. Enrollment into an OLE study typically follows enrollment into a randomized, blinded, well-controlled main study. Participants are usually informed at the time they are recruited into the main study that they may elect to enroll in an OLE study after completing the main trial. The stated objective of most OLE studies is to obtain long-term safety and tolerability data.
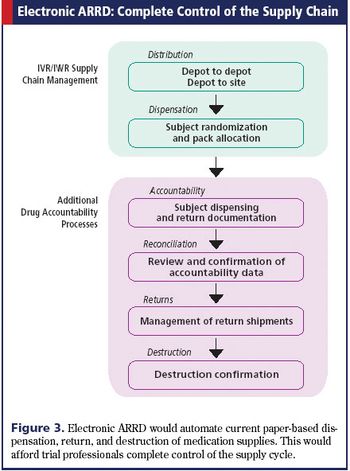
Applied Clinical Trials
Electronic solutions can enhance the efficiency of tracking drugs throughout a clinical trial.

Applied Clinical Trials
Right now the industry has a chance to reclaim its good name and recapture the public's respect for the power of what it can do.