
Applied Clinical Trials Supplements
A new reference guide clarifies uncertainty surrounding this sometimes misunderstood document.

Applied Clinical Trials Supplements
A new reference guide clarifies uncertainty surrounding this sometimes misunderstood document.
Applied Clinical Trials Supplements
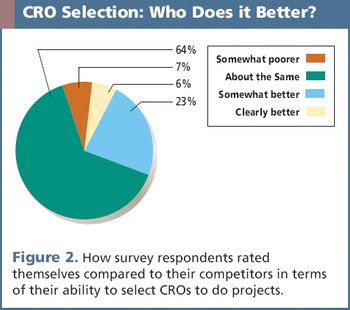
A recent survey uncovers key criteria that influence a sponsor's decision when selecting a CRO.

Applied Clinical Trials Supplements
A list of the most influential events affecting the clinical trials industry over the last 30 years.

Applied Clinical Trials Supplements
Results reveal insight into the roles, activities, pressures, and priorities of study coordinators.

Applied Clinical Trials Supplements
A system of checks and examinations that helps ensure the quality of clinical trials.
Applied Clinical Trials Supplements
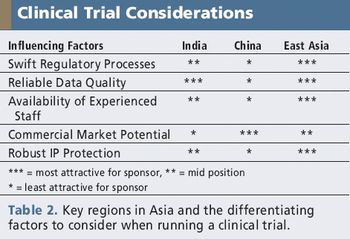
Rapid recruitment, potential cost savings, and investigative sites are just a few of the factors attracting sponsors to the region.