A Review of Critical Issues Detected Via Central Statistical Monitoring
Analysis assesses the relative percentage of quality issues detected via SDM that clinical study teams considered critical.

Statistical data monitoring (SDM), a key component of central monitoring, aims to detect emerging quality issues at investigative sites within ongoing clinical trials in an unsupervised manner by applying multiple statistical tests to each clinical variable collected in the trial. The statistical tests used help to assess data trends over time, data variability within and between patients, data reporting rates, mean values, and a number of other data attributes. All of the patient data (and audit trail data) collected in a clinical trial can help to expose issues in trial conduct and data reliability and are, therefore, worth checking. However, some of the patient data is considered more critical than others—usually because they directly support key efficacy and/or safety endpoints in the study—and atypical trends detected on those variables are, thus, also considered more critical to study teams.
We were interested in assessing the relative percentage of issues detected via SDM that study teams considered critical, for each of the most commonly collected data domains. The results presented here are compiled from studies using the CluePoints risk-based quality management (RBQM) platform, and we selected only studies monitored on the platform since the second half of 2019 in order to focus on the most current trends. Atypical test results are formalized by the study team into “risk signals,” in order to document all follow-up activity on the risk and ultimately to confirm whether an actual issue was identified. Only risk signals marked as actual issues by the study teams were included in the results, and only data domains for which at least five distinct studies and 50 confirmed issues existed. This comprises a total of 4,857 confirmed issues coming from 265 studies contributed by 32 different organizations.
Globally, 47.7% of the confirmed issues detected via SDM were considered critical. The five domains with the highest percentages of critical issues are related to important efficacy and/or safety assessments. The highest rate of critical issues was found with tumor response (87.7%), followed by adverse events (81.8%), immunogenicity specimen assessments (74.5%), disease response (72.3%), and clinical events (70.3%). It is also apparent that the bottom four domains (concomitant medications, subject visits, demographics, and medical history) are less likely to include critical data (see Figure 1).
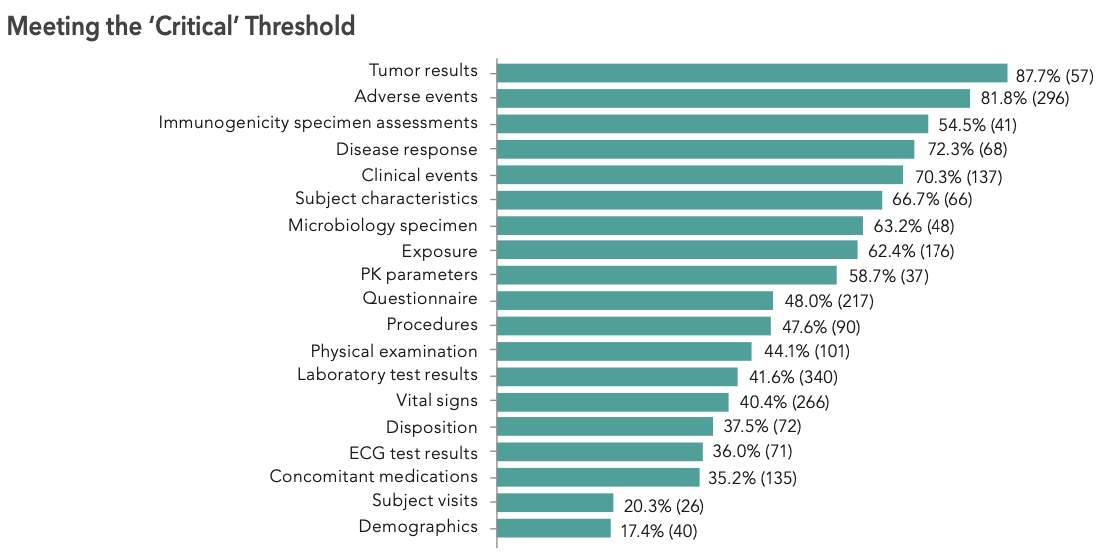
There might be multiple reasons why domains such as tumor response are not 100% of the time related to critical variables. First, some of those datasets or variables might be exploratory and, thus, not considered a primary or secondary endpoint. Second, within an electronic case report form, not all collected information have the same weight.
The issues identified via SDM are primarily systemic in nature, which makes them generally more impactful than issues identified by traditional quality oversight methods. This analysis reveals that almost half of the issues detected by SDM are considered critical in nature—reinforcing the essential importance of central monitoring and SDM in quality oversight.

Unifying Industry to Better Understand GCP Guidance
May 7th 2025In this episode of the Applied Clinical Trials Podcast, David Nickerson, head of clinical quality management at EMD Serono; and Arlene Lee, director of product management, data quality & risk management solutions at Medidata, discuss the newest ICH E6(R3) GCP guidelines as well as how TransCelerate and ACRO have partnered to help stakeholders better acclimate to these guidelines.