COVID-19 and Its Impact on the Future of Clinical Trial Execution
Findings from a Tufts study examining the effects of COVID-19 on clinical trials.
The COVID-19 pandemic caused major disruptions to clinical trial execution in the U.S., impacting key stakeholders across the industry. Investigative site capabilities experienced upheaval, driven by staff furloughs, social-distancing protocols, financial losses, and concerns over patient safety. Sponsors, CROs and other organizations that support drug development shifted to remote working environments. An estimated 80% of non-COVID-19 trials were stopped or interrupted as a result of the COVID-19 pandemic.1 An April 2020 study suggests investigative sites demonstrated flexibility and ingenuity in adopting new approaches in order to cope with challenges presented by COVID, with over half of investigative sites transitioning to virtual approaches to interact with patients. More recently, follow-up studies performed in August 2020 have identified persistent impact of COVID, with over 60% reporting an “average” or greater level of impact on ongoing trials and initiation of new trials. Respondents specifically highlighted challenges in patient enrollment and recruitment.2
Recent FDA guidance (March 2020 and updated July 2020)3 acknowledged that the impact of COVID-19 may require companies conducting clinical trials to consider virtual patient visits or put new processes in place regarding their current protocols. Therefore, there are now more opportunities for using remote healthcare including conducting virtual or decentralized trials, site-less clinical trials and use of other non-traditional approaches that do not involve in-person visits. This shift, driven by the effects of the pandemic, prompted the team at the Tufts Center for the Study of Drug Development to conduct a qualitative study researching the impact of COVID-19 on clinical trial execution.
The study examined the effects of COVID-19 on clinical trials by gathering insights and perceptions from clinical operations professionals at pharmaceutical companies in the United States. The goal was to understand the modifications that organizations have made in response to the pandemic, especially with regard to remote or virtual approaches. In addition, the study explored how companies have approached planning and implementing remote and virtual approaches to trials, as well as their experience and lessons learned. Last, Tufts researchers sought to understand initial perspectives on how COVID-19 will impact trial execution in the long-term.
A total of 25 interviews with pharmaceutical and biotechnology executives within the clinical operations department from unique companies were conducted using Zoom. All executives worked in the United States and played a role in U.S. clinical operations. Participants were selected from Tufts CSDD proprietary lists of contacts who work in clinical roles and through other collaborations on prior studies or from professional conferences and meetings. Participants were recruited via email from June to early August and have extensive experience conducting clinical trials across diverse therapeutic areas. Interviews were 30 minutes in duration and were conducted by Tufts CSDD researchers using a comprehensive discussion guide encompassing the following areas: (1) COVID-19 Impact on Ongoing Trials, (2) Organizational Preparedness, (3) Trial Amendments and Remote Technologies, (4) Role of Regulatory Agencies (5) Role of Third-Parties, (6) Long-term Impact of COVID-19.
Respondent characteristics
Interviews were conducted with U.S.-based staff at 25 organizations comprised of pharmaceutical, biotechnology, and non-profit research institutions. There were 15 large organizations represented and 8 mid-sized or small companies based on R&D spending.* Two were non-profit organizations. These were included given they often sponsor government-funded clinical research.
Impact of COVID-19 on ongoing trials
Eleven organizations halted ongoing trials and enrollment for at least one month. Shutdowns were most frequently driven by concerns that patients would not be able to safely travel to and receive care at research centers. These concerns were especially poignant in indications that were considered “high-risk” for COVID infection (e.g., cardiology, pulmonology, immunology) or indications where patients had difficulty traveling or for example, had neuromuscular and/or neurodegenerative conditions. In these cases, studies often resumed only once patient safety was assured and R&D organizations had amended protocols to include suitable pandemic contingency plans. An interviewee discussed the impact of COVID on their ongoing trials:
Another 12 organizations reported moderate impacts on ongoing trials, most frequently citing delayed enrollment as the primary challenge related to COVID. Several factors may have reduced impact on ongoing trials. Trials in indications such as dermatology and psychiatry may have faced less severe disruption, given more frequent use of telehealth in clinical practice prior to COVID or relative ease of transitioning collection of outcome measures to remote methods (e.g., measuring skin lesion size over telehealth). Additionally, organizations that demonstrated a higher level of pre-COVID preparedness or greater organizational agility tended to report lower impact. Finally, organizations with modest impact often reported partial, geographically isolated closures or understaffing at select sites, rather than full shutdowns reported by those experiencing greater impact. The impact on an organization’s investigative sites is described by a respondent:
Most R&D organizations that reported some level of COVID impact suggested that the ability of sites to respond to the pandemic was a key indicator of the level of impact. Given COVID-19 outbreaks occurred regionally in the U.S., differences in site response emerged based on location and time period. Respondents identified four key factors that tended to influence sites’ abilities to respond to the pandemic. First, staffing furloughs were often site-dependent, and often resulted in delays in data processing, as well as scheduling and execution of patient visits. Additionally, many patients felt hesitant to visit research centers, especially in harder-hit regions, with select organizations citing higher rates of patient withdrawal from studies. Sites with poor or non-compliant IT faced significant challenges pivoting to telehealth visits, providing access to EMR to investigators, or completing CRA site visits virtually. Finally, sites set different restrictions based on hospital- and state-imposed protocols (e.g., no lab-only visits, additional PPE needed, etc.) that resulted in potential deviations from protocol or delays.
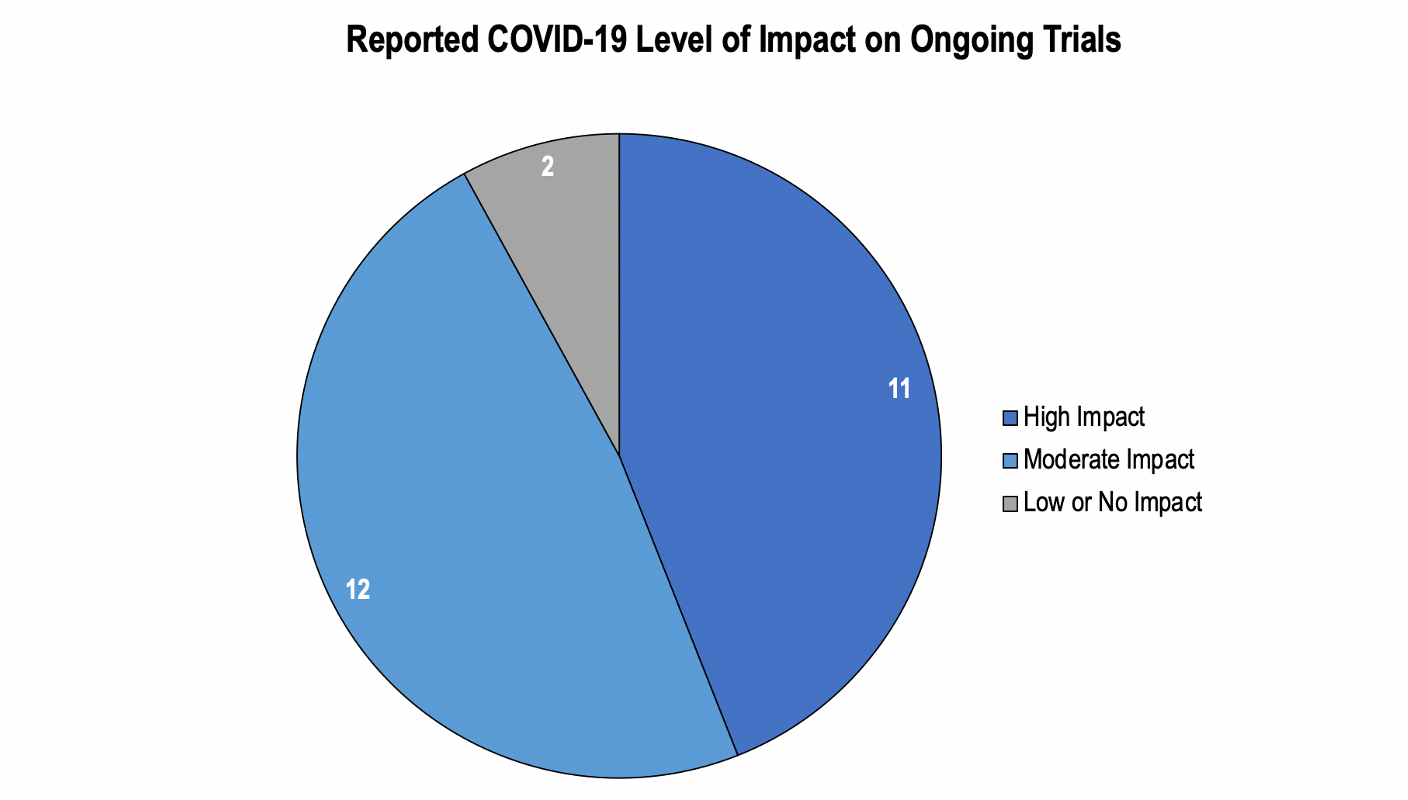
Figure 1. 23 of 25 organizations reported some level of impact on ongoing trials due to COVID-19.
A couple of organizations (n = 2) reported experiencing small or no disruptions. These organizations were often running early stage trials (e.g., Phase I) where clinical follow-ups to identify adverse events could easily be performed over telehealth. An interviewee conducting studies in early phases noted that “there is a fairly large dichotomy between early phase and late phase—the impact for Phase I and Phase II is a little less.” Organizations with less disruptions also were often running trials in disease areas where patients required urgent treatment and/or had few options for treatment (e.g., oncology, rare disease).
Organization response: successes and failures
R&D organizations reported a spectrum of success in responding to the challenges presented by COVID. R&D organizations that self-reported “successful” responses to COVID-19 often demonstrated at least one of four key success factors: organizational agility, prior investment in remote technologies, development of COVID-19 task forces, and/or strong R&D capabilities in Asia-Pacific (APAC) regions.
Five organizations cited organizational agility as a key factor in the success of their COVID response. Smaller, flatter organizations were less hindered by bureaucracy, and were able to rapidly implement changes to studies.Decisions were made at a study-team level and upper management were informed of decision-making. In these cases, it was critical that leadership trusted their employees to execute trial changes without significant oversight.
Another 5 companies were piloting remote technologies and services prior to COVID and reported transitioning more easily to “virtual” trials.Several organizations were piloting telehealth, remote data monitoring, home health, and other virtual approaches in ongoing studies. Comfort with existing remote infrastructure allowed for rapid expansion into indications that did not initially leverage but could benefit from virtual approaches (e.g., measurement of skin lesions over telehealth in dermatology trials). The trend of shifting to a more remote model was explained by an interviewee:
Establishment of a task force was instrumental for larger companies to prepare and rapidly amend trial designs to include remote modalities, with 5 of interviewed organizations reporting implementing such task forces. For larger organizations, the creation of response teams was critical to preparing for COVID’s impact. Organizations that successfully adapted trials to COVID-19 typically had upper leadership that trusted their task force to execute without significant oversight. In select cases, leadership indicated desire to press forward with additional changes to future trials, in order to continue building upon the trend toward virtual trials. The COVID-19 task force typically encompassed representatives from all key trial functions. Experienced team members with backgrounds in operations, regulatory, and data management were all required for implementation of novel remote technologies. Following general guidelines from the COVID-19 task force, study leads often were responsible for managing direct COVID-19 impact. Study leads needed to consider each investigative site separately, given variable COVID-19 activity across regions in the U.S. Task forces that took a study-by-study and site-by-site approach reported greater success than those that attempted a “uniform” approach. The importance of a relatively independent task force that could communicate with study teams and leadership was explained by two interviewees:
A minority of organizations (~2) reported leveraging their organizations’ R&D capabilities in China and Japan, who had early COVID experience, to prepare ongoing studies in the U.S. Select global R&D programs indicated that their colleagues in China and Japan warned them about the impact COVID may have on trial execution, which allowed them to prepare for COVID and begin to develop protocols in case sites were shut down. Of note, this was a benefit that was not reported consistently by global R&D organizations and may be dependent on specific colleague-colleague relationships.
Adoption of remote monitoring technologies and decentralized approaches
Interviewed stakeholders implemented a wide range of novel remote monitoring technologies and decentralized approaches to make trials more patient-centric in the COVID era.
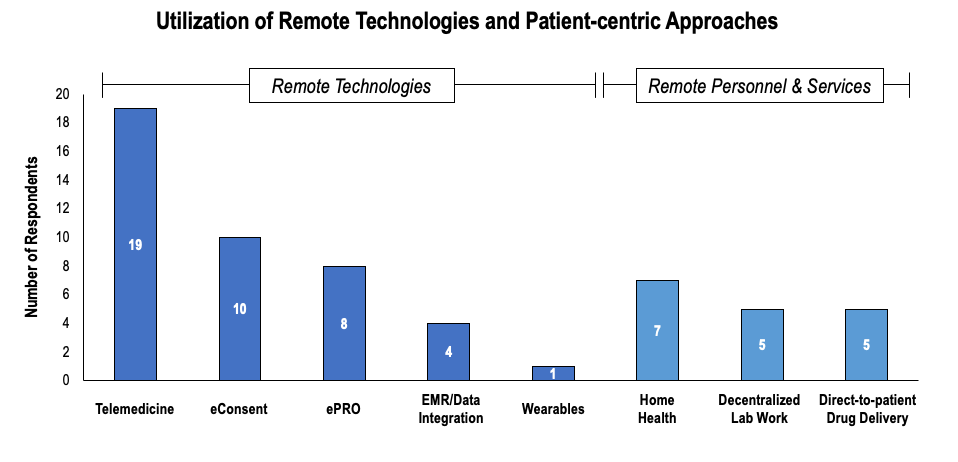
Figure 2. Telemedicine and eConsent were the most frequently utilized remote technologies, while home health was the most frequently utilized remote service.
Telemedicine and eConsent were the most frequently mentioned technologies adopted during COVID-19. Telemedicine was implemented by the majority of respondents, with 19 interviewed organizations reporting use of telehealth in ongoing trials. Telemedicine was most frequently used for routine follow-ups to identify adverse events, especially in Phase I studies. Telehealth was also used to measure select objective outcome measures (e.g., lesion size in dermatology or plaque psoriasis). Though investigators recognized the potential to collect other measures via telehealth (e.g., depression scales), they expressed concern that the method is not validated and requires proof of concept before regulators would accept data generated through telehealth. eConsent was used by 10 companies and was perceived to be one of the simplest innovations to implement. Respondents highlighted the potential for eConsent to facilitate communication with patients, by leveraging novel modalities such as video explanations, quizzes to assess understanding, etc. Interviewees offered their insights on remote technologies:
ePRO was the next most frequently utilized technology, with eight companies citing use. Notably, ePRO was already used in ongoing trials prior to COVID. However, in an effort to further reduce patient burden, research organizations opted to allow patients to use their own devices.
Use of data integration was perceived to be highly useful to R&D organizations, given R&D organizations could access trial data in real-time. Additionally, utilization of EMR provided opportunities for R&D organizations to supplement clinical research data, as potential alternatives to monitor adverse event and clinical care patterns. However, only select companies (n = 4) actually leveraged real-time integration of trial data or EMR during COVID. Several respondents cited challenges with implementation of integrated EMR, given poor investigative site IT infrastructure, investigative site preferences, or challenges with CRO staffing or capability to visit sites. Privacy restrictions, interoperability and compatibility challenges represent additional key barriers to utilization of these technologies. One interviewee discussed the barriers that have emerged in monitoring due to COVID:
A number of respondents expressed interest in the future use of integrated EMR, and suggested they were actively seeking CRO partners to implement the technology once sites reopened. In addition to novel technologies, respondents adopted a range of approaches to decentralize trials and minimize patient exposure to investigative sites in the face of COVID-19. Overall, nearly half of respondents implemented at least one approach to decentralize ongoing trials.
Approximately seven organizations implemented some method of home health in their ongoing trials to ensure patients that were unable or unwilling to travel received IV medications and were able to provide blood samples. Rare disease trials reported greater use of home health, given the long distances patients may have to travel to access the investigative site and the need for frequent IV infusions. However, investigators also noted that home health was “hit-or-miss,” given some patients resided in locations inaccessible to home health while others refused to allow nurses in their home due to concerns with the spread of COVID-19. “Home care and direct-to-patient drug delivery are steps to move towards a more patient-centric trial design,” commented one interviewee. Direct-to-patient drug delivery was implemented by five companies and perceived to be a relatively low-risk method to transition trials to be more patient-centric, especially for oral agents with safer expected toxicity profiles.
Decentralized lab work was utilized by five organizations. Many sites did not allow “lab-only” visits during COVID-19, presenting challenges to collecting biomarker-related outcomes in oncology, cardiology, diabetes, etc. Investigators allowed participants to have blood work done at local labs, and in select cases provided co-pay cards to cover the cost of lab work (e.g., via Scout). Decentralized lab/blood work was perceived to be highly effective for oncology and rare disease patients, especially for those that had to travel longer distances to investigative sites.
Overall, remote technology adoption was more extensive than adoption of remote services. One might speculate that remote technology adoption has been more extensive not only because companies had prior experience with these technologies (e.g., piloting) but also because remote personnel and drug delivery require more lead time to set up, more extensive logistics and certification planning.
Roles of regulators
More than half of organizations interviewed (13 companies) cited leveraging FDA guidance at the start of COVID-19 to adapt and amend clinical trials. Interviewees cited published guidance on home-dosing and telemedicine as being especially helpful at the start of COVID. Interviewees also perceived the FDA to be responsive and accepting of the need for flexibility, so long as the integrity of trial data was preserved, and patient safety was ensured. Several interviewees spoke directly with the FDA and rated the experience as positive and informative.
However, interviewees also noted that regulators’ stances on how or if they would accept data that had been gathered in amended trials was unclear, and how the utilizations of remote technologies would impact filing. Specifically, several interviewees expressed concerns that select outcome measures may not be accepted without proof of concept (POC) demonstrating that collection via remote technology was the same as in a physician practice, given current FDA standards on how data should be measured.
Perspectives on long-term impact of COVID
Ultimately, 20 of the 25 interviewees believed that COVID-19 would permanently accelerate use of remote technologies in clinical trials. Most interviewees perceived increased use of remote technologies as a “silver lining” to COVID-19. Respondents often cited telehealth, e-consent, and EMR/data integration as the approaches that would most likely continue to persist past COVID. Additionally, respondents mentioned that they would aim to streamline the data collected from trial protocols, seeking to provide greater flexibility to investigators while removing “nice-to-have” outcome measures from protocols. Meanwhile, high upfront investment cost and unclear regulatory stance drove several interviewees to predict that trials would return to “normal” post-COVID-19. This perspective was especially clear for oncology and rare disease, where stakeholders believed in-person care was critical. Ultimately, most interviewees acknowledged that while use of remote technologies had been accelerated by COVID, use would not persist without concerted effort from industry and regulators to continue momentum. Change would focus on convenience for the patient as highlighted during an interview:
Challenges to adoption of remote technologies
Interviewed stakeholders identified several barriers to the adoption of remote technologies and services. Several interviewees (n = 5) suggested that preference for care at investigative sites would delay implementation of remote technologies. Patients, especially in rare diseases, may prefer to receive care at investigative sites from an in-person key opinion leader (KOL) rather than via telehealth. Others (n = 4) suggested site-specific protocols would hinder adoption. Select sites demonstrated resistance to adoption of new technologies or had specific IT protocols that were barriers to adoption of novel approaches (e.g., poor security delayed remote EMR access). Finally, 8 companies perceived regulators to pose a significant barrier to adoption. Interviewees believe there is low incentive to adopt new technology, given high early investment cost and unclear guidance from the FDA on how they will interpret these technologies during filing. Thus, a firm stance is needed from the FDA to promote utilization of remote technologies post-COVID.
Steps to continuing progression to “patient-centric” trials
Industry, regulators, and investigative sites will need to take several steps to cement the role of virtual approaches and promote patient-centricity in future clinical trials. A strong regulator stance on the use of virtual and remote technologies in clinical trials is necessary to promote adoption by industry. Currently, lack of clarity from regulators on how remotely-generated data will be accepted leads to hesitancy to adopt novel technologies. Published FDA guidance expressing standards for e-Consent, telehealth, and other tools or decentralized approaches may be necessary for organizations to invest in remote technologies. Conversely, POC may be required for regulators to publish such guidance, resulting in a dilemma for companies.
Additionally, it is critical that all stakeholders including patient advocacy groups and investigative sites be on board in order to move decentralized models forward. Several interviewees perceived that select patients enroll in trials to get “better” care at academic centers. Patient perspectives must be incorporated into trial design in order to balance the patient-centric and cost-related benefits of remote technology with the preferences of patients. Additionally, investigative sites may not have the budget nor the expertise to implement novel remote technologies (e.g., EMR integration, telehealth, e-consent). Industry may need to support sites by investing in technologies and trainings to ensure standardization across investigative sites.
Stephen Le Breton, Research Analyst; Mary Jo Lamberti, Professor and Associate Director; Adam Dion, Research Anlayst; all from Tufts CSDD; Ken Getz, MBA, is Deputy Director and Research Professor, Tufts CSDD, and Chairman of CISCRP, both based in Boston, MA. Email: kenneth.getz@tufts.edu
References
- Dorn, A. V. (2020). COVID-19 and readjusting clinical trials. The Lancet,396(10250), 523-524. doi:10.1016/s0140-6736(20)31787-6
- Medidata. (2020, August 18). COVID-19 and Clinical Trials: The Medidata Perspective. Retrieved from https://www.medidata.com/wp-content/uploads/2020/08/COVID19-Response8.0_Clinical-Trials_2020824_v1.pdf
- United States of America, Food & Drug Administration. (2020). FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Public Health Emergency. FDA.
Footnotes:
*Categories of R&D spending based on large companies (R&D >$1Billion); mid-sized (R&D $100 Million - $1 Billion); and small (R&D < $100 Million)
Improving Relationships and Diversifying the Site Selection Process
April 17th 2025In this episode of the Applied Clinical Trials Podcast, Liz Beatty, co-founder and chief strategy officer, Inato, discusses a number of topics around site engagement including community-based sites, the role of technology in improving site/sponsor relationships, how increased operational costs are impacting the industry, and more.
Putting Collective Insights Into Action to Advance Cancer Care: Key Examples From ASCO 2025
June 27th 2025At ASCO 2025, clinical operations leaders gained critical insights into how AI tools, bispecific antibodies, and evolving treatment paradigms are reshaping trial design, endpoint selection, and patient stratification.