Ukraine War's Impact on Clinical Research: Evidence from Key Risk Indicators
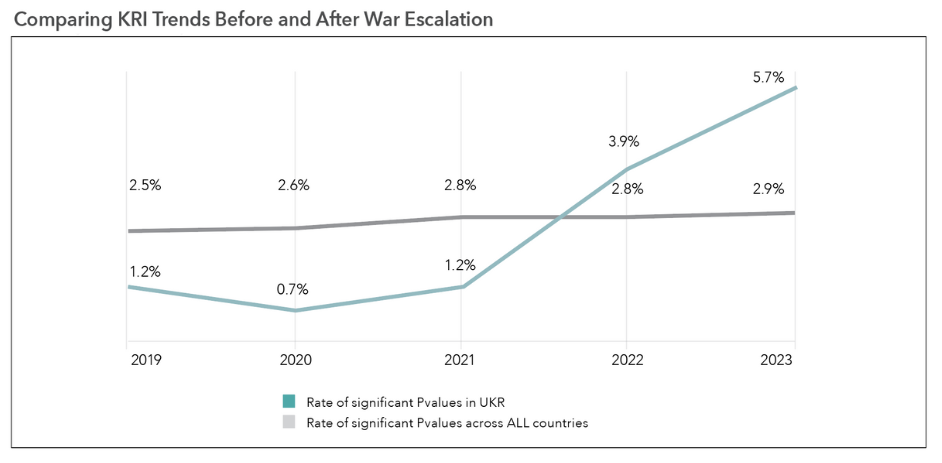
Overall rate of significant KRI results for sites located in Ukraine has consistently risen since 2021.
Key risk indicators (KRIs) are commonly used as an important component of central monitoring to enable study teams to detect emerging quality issues of interest during a clinical trial. Given the large volume of KRI-related information now available for global clinical trials on the CluePoints risk-based quality management (RBQM) platform, we decided to take the opportunity to assess to what degree this KRI data provides evidence of the disruption of the war in Ukraine to clinical research being conducted there.
We assessed the impact of the war in Ukraine across 10 commonly used KRIs in drug development, covering several categories of study risk (compliance, timeliness of data reporting, enrollment and retention, and safety).
KRI results from investigative sites located in Ukraine were compared with KRI results from sites in all countries globally. The analysis utilized the following scope of information:
- Ukraine: 63 studies, 460 sites, and 27,631 individual KRI results
- Globally: 440 studies, 27,591 sites, and 1,740,642 individual KRI results
Individual KRI results on the CluePoints RBQM platform include a statistical p-value that measures the degree to which a given site is different than other sites in the same study for the given KRI metric. A p-value < 0.05 is used to identify sites that are significantly different and therefore considered “at-risk” for the KRI. We computed the overall percentage of KRI results that were significant (p-value < 0.05) in Ukraine vs. globally on an annual basis starting with 2019. This allows us to compare the KRI trends in Ukraine both before and after the escalation of the war in February of 2022.
The overall rate of significant KRI results for sites located in Ukraine raised from 1.2% in 2021 to 3.9% in 2022 and 5.7% in 2023, while the rate across all countries remained stable around 2.8% during the same time period.
This is clear evidence of a significant disruption to clinical trial operations in Ukraine during the current crisis.
In particular, the current rate of identified issues (5.7%) is close to five times greater than it was in 2021, and double the global rate.
Figure 1. Rate of significant p-values in Ukraine vs. all countries.
Source: CluePoints
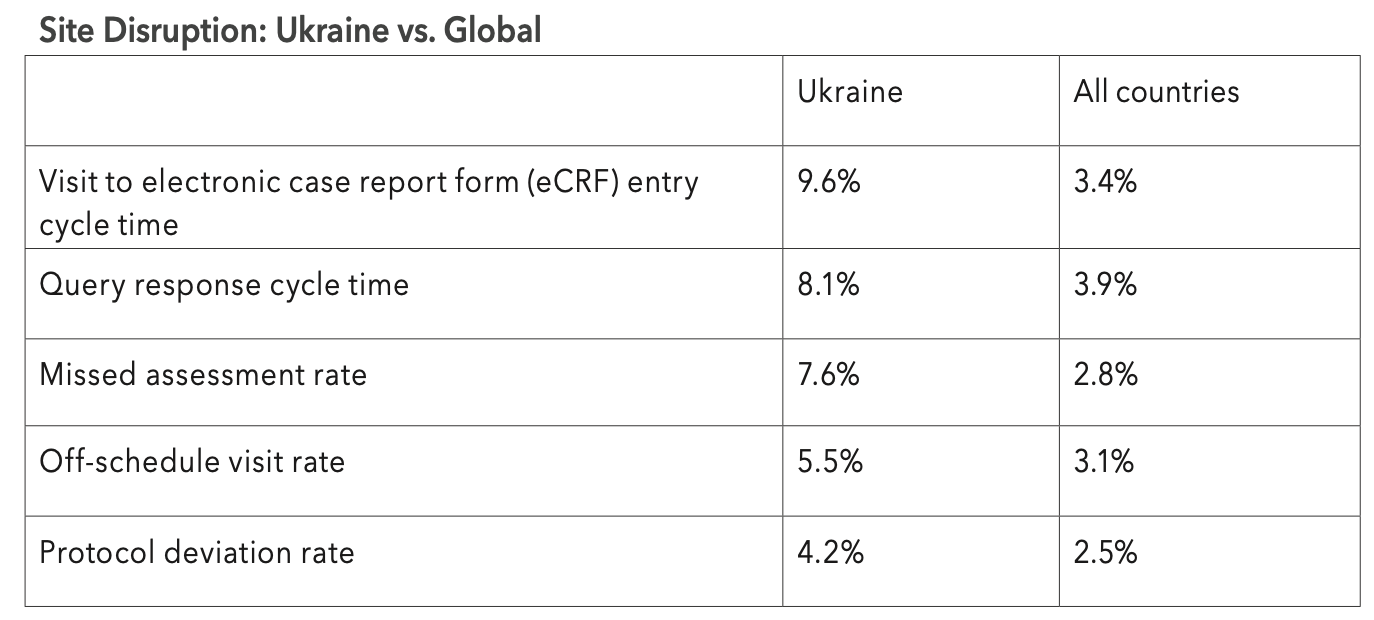
The five most significantly impacted KRIs in Ukraine are presented in Table 1 below, and are focused on issues with protocol compliance and timeliness of data reporting, as one might expect.
Table 1. The top five key risk indicators with the highest rate of significant p-values in Ukraine since 2022.
Source: CluePoints
Whether crises take the form of a pandemic or a regional war, the successful ongoing conduct of clinical research relies greatly on an imperative to support public health. Risk planning and risk control strategies should be implemented to ensure that patients get the care they need and that clinical trial data remain reliable.
Steve Young, chief scientific officer, and Sylviane de Viron, data and knowledge manager; both with CluePoints

Unifying Industry to Better Understand GCP Guidance
May 7th 2025In this episode of the Applied Clinical Trials Podcast, David Nickerson, head of clinical quality management at EMD Serono; and Arlene Lee, director of product management, data quality & risk management solutions at Medidata, discuss the newest ICH E6(R3) GCP guidelines as well as how TransCelerate and ACRO have partnered to help stakeholders better acclimate to these guidelines.