
This recent report explores key factors behind customer satisfaction and other dynamics within the Late Phase market.

This recent report explores key factors behind customer satisfaction and other dynamics within the Late Phase market.
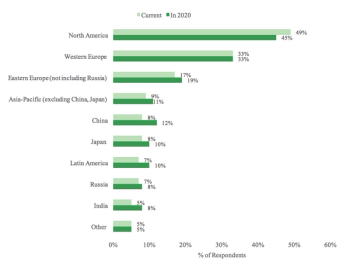
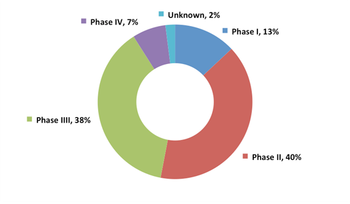
Report shines light on the current state of the Phase II/III clinical trial market and its anticipated direction into 2020.
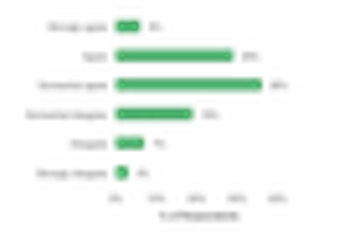
We all need help sometimes. For pharmaceutical companies, this often comes in the form of a reliable and competent contract research organization.
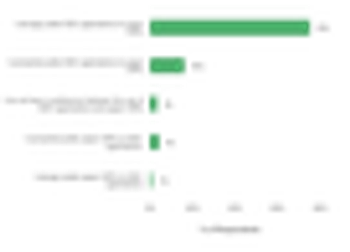
Accuracy in data capture methods has always been and will continue to be a foremost necessity in pharmaceutical clinical trials.
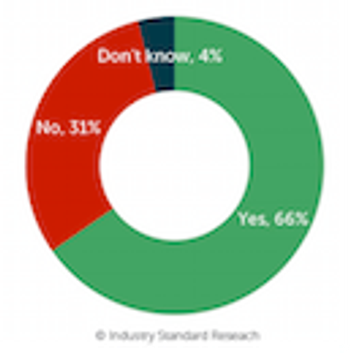
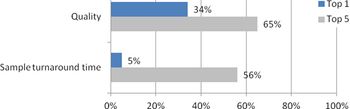
It is well known within the industry that being on a sponsor’s preferred provider list affords a critical leg up to providers hoping to win business. Phase II/III work is no exception. In the 7th edition of Industry Standard Research’s CRO Quality Benchmarking - Phase II/III Service Providers report, ISR digs a bit deeper into the dynamics of preferred provider lists and how these lists affect CRO selection for Phase II/III studies.
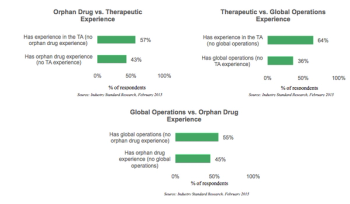
Despite the substantial difficulties associated with orphan drug trial recruitment, this segment of the drug development market is booming.

Spoiler alert: most IRT systems are not meeting user expectations in terms of eClinical integration.
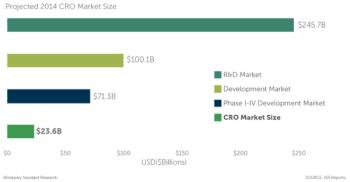
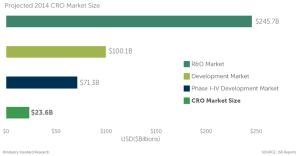
Those of us who have spent considerable time working in the CRO industry have our own version of a "constant" and that is constantly asking the same question: What is the size of the CRO market?

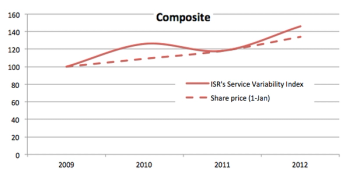
With ISR?s newest market research report (?Risk-based Monitoring: Industry Guidance on Adoption, Use, and Outsourcing?) Mr. Schafer is back to draw comparisons and share data from their latest survey.
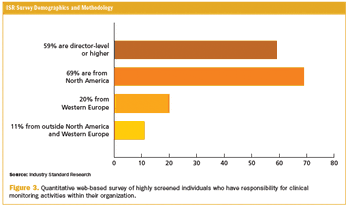
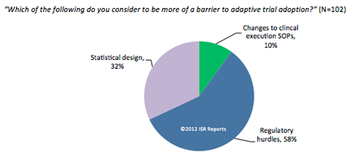
Current commercial forces are working to accelerate the adoption of adaptive monitoring designs.

Published: February 3rd 2014 | Updated:

Published: June 20th 2013 | Updated:
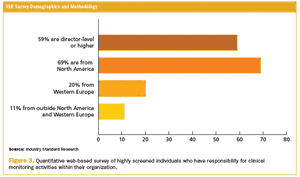
Published: April 1st 2013 | Updated:
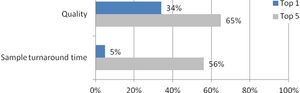
Published: November 12th 2012 | Updated:
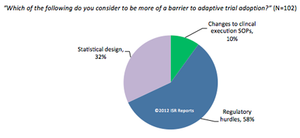
Published: December 3rd 2012 | Updated:
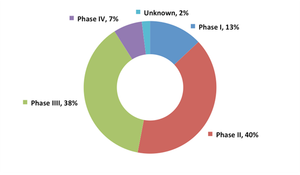
Published: October 22nd 2012 | Updated: