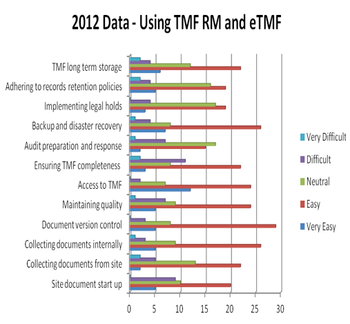
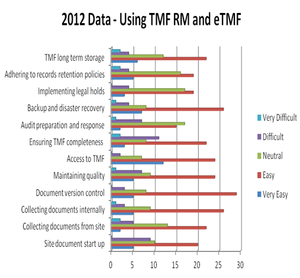
One of the many activities across the clinical development cycle that is non-negotiable is the creation, collection, management, and storage of the documents that are contained in the Trial Master File.
Karen Redding, Global Business Development Director of Phlexglobal, is the Co-Chair of the TMF Reference Model Group and can be reached at kredding@phlexglobal.com

One of the many activities across the clinical development cycle that is non-negotiable is the creation, collection, management, and storage of the documents that are contained in the Trial Master File.
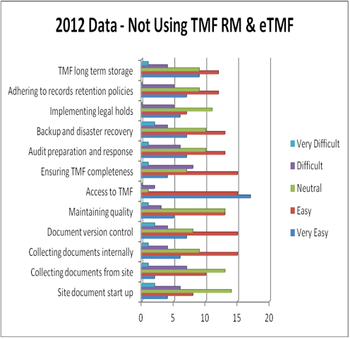
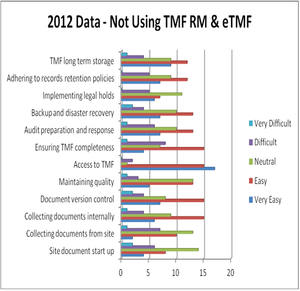
The research and development process continues to be long, complex and costly. Sponsors are challenged with filling pipelines, conducting complex global clinical trials and complying with requirements for additional safety and efficacy data.
Published: September 17th 2013 | Updated:
Published: May 20th 2014 | Updated: