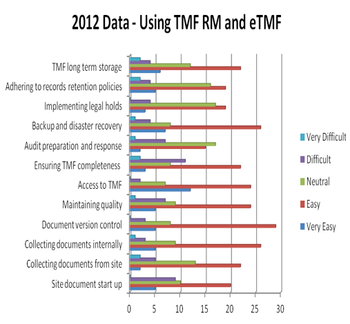
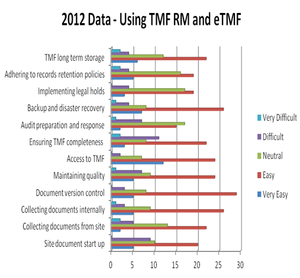
One of the many activities across the clinical development cycle that is non-negotiable is the creation, collection, management, and storage of the documents that are contained in the Trial Master File.
Maryanne Quinn, President, Integrated Submission Strategies (ISS), is Chair of the TMF RM Communication Team and has over 25 years of experience in the Life Sciences Industry and can be reached at ISS.Quinn@verizon.net

One of the many activities across the clinical development cycle that is non-negotiable is the creation, collection, management, and storage of the documents that are contained in the Trial Master File.
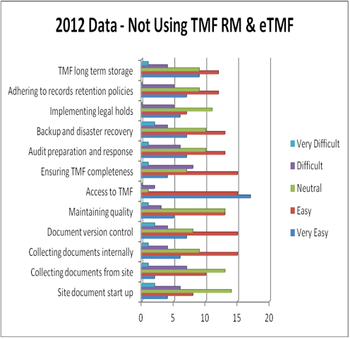
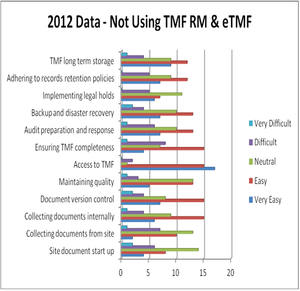
The research and development process continues to be long, complex and costly. Sponsors are challenged with filling pipelines, conducting complex global clinical trials and complying with requirements for additional safety and efficacy data.
Published: September 17th 2013 | Updated:
Published: May 20th 2014 | Updated: