
Study Start-Up
Latest News

Latest Videos
More News

The activation of each clinical trial involves hundreds of tasks, along with many dependencies and handoffs, some of which may be automated, but many require manual processes.

Amplifying the size and diversity of participant pools in clinical trials enhances statistical power and increases the generalizability of study findings.

Optimizing feasibility through increased data collection.

Similar to a world-class symphony, a curated site start-up model can accentuate its individual components and lead to swift starts for clinical trials.

Sujay Jadhav, global vice president, study start-up, Oracle Health Sciences, spoke with ACT on how study start-up has been impacted by the pandemic and what its future holds beyond COVID.

With a major wave of post-COVID trial starts on the horizon, the industry’s need to accelerate clinical research through study start-up is intensifying.

Bayesian methods bring flexibility and speed to clinical trial design and analysis, and with increased access to the necessary computational power, are transforming today’s clinical research.

Why? Because more often than not, you do not want to accrue only one participant to a clinical trial.
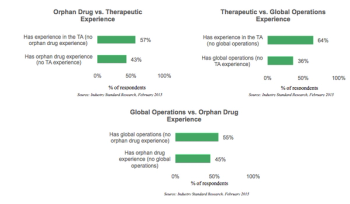
Despite the substantial difficulties associated with orphan drug trial recruitment, this segment of the drug development market is booming.




