Articles by Venkat Sethuraman, PhD

FDA commissioner Scott Gottlieb criticized pharmaceutical companies and clinical research organizations for being slow to adapt innovations in clinical research, claiming that the business model adopted just isn’t compatible with the kind of changes that certain innovations can enable.

These seven key building blocks for success are outlined to help companies develop and implement a data-and-analytics-driven approach to clinical trial feasibility.
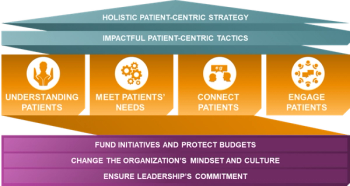
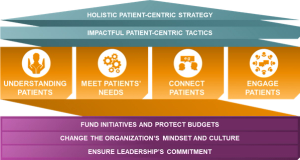
ZS Associates specify the four pillars of patient centricity that can help the demands and complexities of clinical trials.