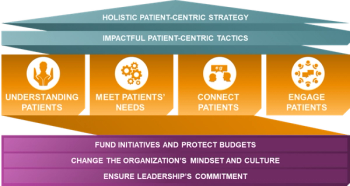
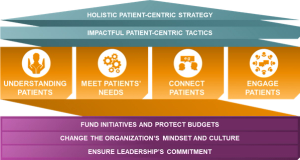
ZS Associates specify the four pillars of patient centricity that can help the demands and complexities of clinical trials.

ZS Associates specify the four pillars of patient centricity that can help the demands and complexities of clinical trials.
Published: October 19th 2017 | Updated: