
DEI
Latest News
Latest Videos

More News

The FDA's guidance is part of a broader effort to modernize clinical trials, improve efficiency, reduce participant burden, and expand access, particularly for underrepresented populations and those in geographically or economically constrained areas.

Participants touch on the different aspects of FDA's recent Diversity Action Plan guidance and how industry must be held accountable for implementing change.

New paper features insights from nearly 100 experts on four key barriers to clinical trial access.

How the inclusion of measures centered on net treatment benefit can drive an effective multifaceted approach.

With universal adoption of health literacy best practices slow going over the years, advocates are redefining the term to encompass much more of what health-related communication requires beyond simply words.

In the second part of this roundtable discussion, participants highlight best practices for reaching underrepresented populations and increasing their awareness of clinical trials.

Understanding how the dynamics between the two play hand in hand in influencing patient recruitment and retention.

In the first part of this roundtable discussion, a panel of experts introduce themselves and highlight the challenges industry is seeing with underrepresentation of racial and ethnic groups in clinical trials.

In this video interview, Jay Park, founder & scientific lead, Core Clinical Sciences, discusses how risk-based monitoring can alert sponsors of falling short of enrollment goals.

In this video interview, Rebecca Metcalfe, principal scientist, patient-centered research, Core Clinical Sciences, highlights the consequences of focusing solely on enrollment targets.

An overview of key trends in communication, logistical preferences, and demographic influences that shape patient engagement and retention in clinical trials.

In this video interview, Jay Park, founder & scientific lead; and Rebecca Metcalfe, principal scientist, patient-centered research; both with Core Clinical Sciences, share their reactions to the FDA releasing its Diversity Action Plan Guidance.

In this video interview, Jay Park, founder & scientific lead; and Rebecca Metcalfe, principal scientist, patient-centered research; both with Core Clinical Sciences, discuss a recent article they authored on the guidance.

In this video interview, Jimmy Bechtel, vice president, site engagement, SCRS, discusses some key initiatives around decentralized capabilities and underrepresented patient populations.
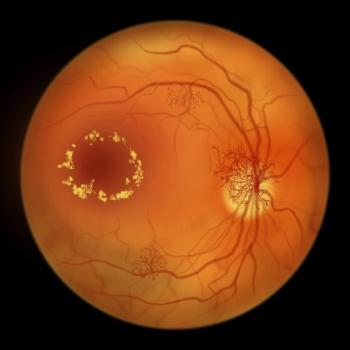
Vabysmo Shows Significant Vision Improvement for Diabetic Macular Edema in Underrepresented Patients
Phase IV ELEVATUM trial results show that one year of treatment with Vabysmo significantly improved vision in underrepresented racial and ethnic groups with diabetic macular edema, supporting the drug's efficacy and safety across diverse populations.

Industry leaders share their perspectives on the guidance and how it will affect sponsors moving forward.

From design and trial start-up to conduct and analysis, there is enormous potential for applications of artificial intelligence within clinical trials to have a profound impact on human health.

In this video interview with ACT editor Andy Studna, Mwango Kashoki, SVP, global head of regulatory strategy, Parexel, discusses how the guidance should prompt better-designed oncology trials and greater diversity.

In this video interview with ACT editor Andy Studna, Mwango Kashoki, SVP, global head of regulatory strategy, Parexel, touches on challenges that may be created by the guidance such as meeting multiple regulatory requirements.

In this video interview with ACT editor Andy Studna, Mwango Kashoki, SVP, global head of regulatory strategy, Parexel, discusses how sponsors will need to plan ahead even further for their oncology trials.

In this video interview with ACT editor Andy Studna, Mwango Kashoki, SVP, global head of regulatory strategy, Parexel, highlights how FDA’s multiregional clinical trials in oncology guidance encourages more diverse site locations and patient populations.

In this video interview with ACT editor Andy Studna, Kashoki, SVP, global head of regulatory strategy, discusses the new draft guidance and its emphasis on proactive planning.
Twice-yearly lenacapavir, a long-acting HIV-1 capsid inhibitor, reduced HIV infections by 96% in the Phase III PURPOSE 2 trial while showing superior efficacy over daily Truvada.

Findings from two Baltimore medical centers presented at the American Society for Radiation Oncology Annual Meeting suggest spiritual themes and distrust may be behind the decline in trial participation.

Cohort study of over 290,000 patients found disparities in cancer stage at diagnosis and survival.













