How High-Quality, Curated Real-World Data from Clinical Data Registries Helps Improve Drug Trials
As clinical trials continue to advance and evolve to include real-world data, their success is becoming ever more dependent on the quality of data.
Image credit: Dmitry | stock.adobe.com

A clinical trial is only as good as how well it is designed. For rare diseases or conditions, specifically, real-world data (RWD) is becoming more essential for determining whether a sponsor’s prospective trial criteria are too restrictive or too broad, which can inform the trial design. For example, excessively restrictive exclusion criteria can make patient recruitment costly and time-consuming, while overly broad inclusion criteria can make it more difficult to generate meaningful data to make the case for a specific therapeutic.
Recently, a sponsor sought to conduct a Phase II anonymized clinical trial for an ocular drug to treat patients with a rare and painful complication resulting from refractive surgery procedures, such as LASIK and photorefractive keratectomy. No clinical trials had yet been done on patients with this specific adverse effect (AE).
The trial sponsor had developed profiles of characteristics and symptoms that could be used to delineate the patient population. These factors included the types of drugs patients had been taking to treat the AE, their level of discomfort, who manages their conditions, and other variables.
However, the trial sponsor lacked the data to quantify the prevalence of this condition and had no insight into how many patients experienced this AE following refractive surgery. Additionally, while the symptoms had been mentioned anecdotally, ophthalmologists also struggled to estimate the size of this unique patient population.
In part, this was because refractive surgery often is paid for by patients out-of-pocket, and therefore, not well captured in claims data. Complicating matters further, physicians may use heterogeneous terminology and shorthand when documenting refractive surgery procedures, resulting in little uniformity. Finally, documentation of this type of pain in electronic health records (EHRs) is inconsistent, making it difficult for practices to find eligible patients.
The lack of visibility made it a great challenge for the trial sponsor to quickly identify, recruit, and enroll enough patients with this rare AE to successfully launch and complete a Phase II trial. It was critical for the sponsor to gain access to quality RWD to move their development program forward.
Enter the IRIS Registry
To help find more qualified participants for the clinical trial, the sponsor collaborated with Verana Health to leverage curated RWD data from the American Academy of Ophthalmology (Academy) Intelligent Research in Sight (IRIS) Registry. Established in 2014, the IRIS Registry is one of the largest specialty society clinical data registries in all of medicine.
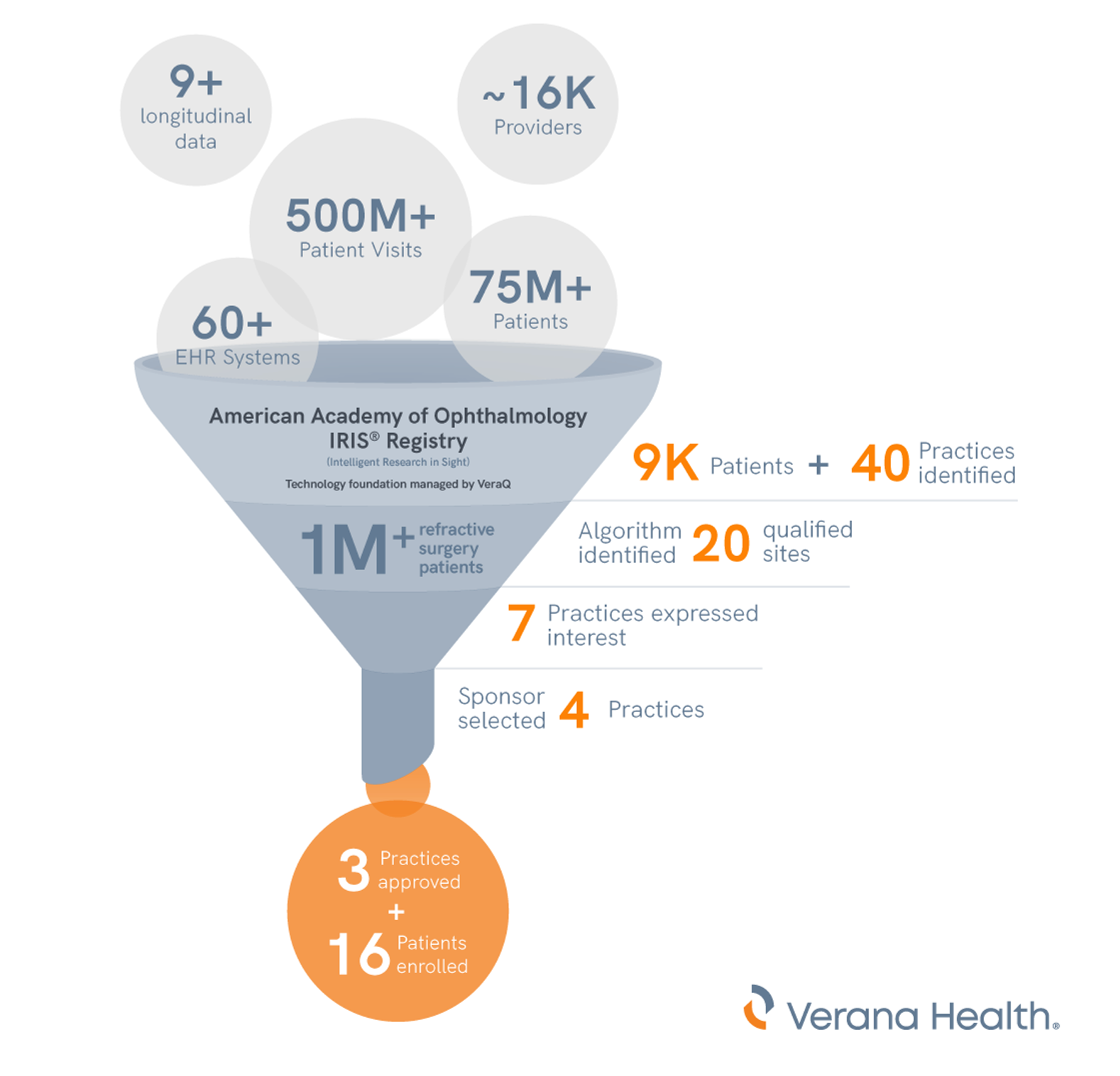
The IRIS Registry, which has a technology foundation managed by the VeraQ population health data engine, contains an enormous amount of RWD. It provides researchers with access to a longitudinal database covering nearly 10 years of de-identified patient demographics, patient medical and ocular history, clinical examination findings, diagnoses, procedures, medications, and more. These data are amassed from 490 million billable visits, 79 million unique de-identified patients, and approximately 16,000 ophthalmologists and other eye care providers across the United States.*
Within the IRIS Registry, much of the relevant symptoms of chronic pain following refractive surgery is found in unstructured form (primarily clinicians’ notes). To extract this information in a reliable and scalable manner, clinician-informed artificial intelligence (AI), such as machine learning (ML) and natural language processing (NLP), was used to search through and contextualize unstructured clinician notes within the IRIS Registry to help identify:
- Refractive surgery patients
- Patient-reported pain symptoms (e.g., burning, stinging, stabbing, foreign body sensation or itchy eyes)
- Diagnostic terms describing the patients’ symptoms and pain
- Whether patients were seeing other specialists
This approach—relying on a combination of physician expertise and AI—enabled Verana Health to partner with the sponsor to translate coding and documentation in clinicians’ notes into a clinical understanding of a patient’s journey.
Identifying patients and trial sites
For the study, a cohort of more than one million patients who had undergone refractive surgery was identified using high-quality, curated IRIS Registry data. To further refine the patient cohort, the sponsor added trial inclusion/exclusion criteria, including age and exclusionary ocular diseases, and then searched for cases in which patients reported post-LASIKor other problems following refractive surgery. This helped the sponsor to identify several hundreds of thousands of patients who described specific symptoms following surgery.
From there, Verana Health was able to identify approximately 9,000 patients across 40 different practices who reported the specific symptoms of interest. A custom algorithm developed to identify the most ideal patients from that group narrowed the list of prioritized sites to 20.
By applying rules to narrow down the list of potential study sites—for example, proposed study practices must have 40 or more potential study participants—the algorithm identified the 20 qualified sites. Of the 20 sites, seven expressed interest to learn more. Eventually, the sponsor chose four practices as trial sites, three of which were approved for the trial, and enrolled 16 patients who met the screening criteria in the Phase II study.
The value of quality, curated RWD
Without quality curated data, sponsors often lack information and context to determine whether their trial criteria are too broad or unrealistically restrictive. Curated RWD drawn from the IRIS Registry helped the sponsor select the optimal inclusion criteria to better ensure enough participants could be identified. Quality data also can inform the site selection process, helping the sponsor better ensure that the sites selected for launch will likely yield eligible patients for the study.
The benefits of quality curated RWD extend to medical practices as well. Using an RWD-driven approach in trials can help to facilitate new trial opportunities from sponsors with which a practice may not be familiar. And it can help build pathways to connect patients with rare diseases or limited treatment options to trial sites, when specialty care is needed. Further, when practices can securely access their own data through this process, they are able to identify potential high-quality candidates more efficiently for their trials, which benefits the patient and helps the site manage costs.
RWD is a useful tool to help advance, enhance, and improve the clinical trial process. Importantly, it can also help to increase trial access for patients, which has the domino effect of helping to accelerate trial recruitment and providing a more representative set of participants. A representative set of study participants also helps to promote trial diversity, which is not only in line with the FDA’s Advancing Real-World Evidence Program, but can ultimately help drive equitable therapeutic advancements to help advance patient care across underserved populations.
Conclusion: Curated RWD can help advance clinical trials
Finding patients to enroll in clinical trials using traditional methods—particularly for rare or hard-to-treat conditions—can often be challenging for sponsors and study investigators to find. Additionally, site selection in clinical trials can benefit from data-driven approaches that identify appropriate practices and geographies to serve the patient community.
Using RWD as a starting point often offers an opportunity for trial sponsors and practices to collaborate on research into therapies for rare diseases for which participants may be hard to identify and recruit. Trial sponsors can use curated RWD from medical registries to help identify sites for trial participation, helping them reduce costs associated with opening additional trial sites or extending the recruitment period.
Registry partnerships can be a valuable platform to help support clinical research. This combination of a strong data and technology foundation coupled with a robust physician network helps speed trial recruitment.
As clinical trials continue to advance and evolve to include RWD, their success is becoming ever more dependent on the quality of data. Utilizing clinical data registries—coupled with trusted and scalable curation of that data—can help promote data-driven approaches to fine-tune eligibility criteria and to identify patients and practices. Ultimately, this approach helps to reduce the number of sites needed to fully recruit for a trial, reduce screen-fail rates, minimize recruitment periods, improve diversity and representation, and help sponsors stay top-of-mind in a competitive landscape.
*January 2023 numbers
About the Author
Sujay Jadhav is CEO of Verana Health.
Newsletter
Stay current in clinical research with Applied Clinical Trials, providing expert insights, regulatory updates, and practical strategies for successful clinical trial design and execution.
Managing Side Effects and Dosing in Off-Label GLP-1 Use with Help from Real-World Evidence
July 18th 2025Shipra Patel, global therapeutic area section head, endocrinology, global head, pediatrics, Parexel, explains how real-world data is helping researchers navigate gastrointestinal side effects, dose flexibility, and long-term tolerability in off-label GLP-1 use.
Unifying Industry to Better Understand GCP Guidance
May 7th 2025In this episode of the Applied Clinical Trials Podcast, David Nickerson, head of clinical quality management at EMD Serono; and Arlene Lee, director of product management, data quality & risk management solutions at Medidata, discuss the newest ICH E6(R3) GCP guidelines as well as how TransCelerate and ACRO have partnered to help stakeholders better acclimate to these guidelines.
Effect of AI/ML, Real World Evidence and Master Protocols on Trial Success
July 7th 2025How the application of artificial intelligence, broader use of real-world evidence, decentralized clinical trials, master protocols, and risk-based quality monitoring, together with strong ethical oversight and increased collaboration, are contributing to better healthcare delivery and strengthening the role of clinical research in driving global health progress.