Technology-Enabled Real-World Data and Clinical Research Data Integration in a Clinical Registry Ecosystem for Foundations
Spearheaded by disease foundations, the creation of clinical registries is streamlining data collection and driving informed decisions.
Real-world data (RWD) and real-world evidence (RWE) have become indispensable elements in the current clinical research scenario, intertwining seamlessly with traditional methodologies. Disease foundations are actively spearheading the establishment of clinical registries, across various therapeutic areas, diligently gathering RWD from targeted patient populations. This will aid in driving informed decision-making in healthcare, catalyzing the advancements in clinical research, fostering the development of novel interventions, and shaping public policies and regulations.
It is, therefore, important to understand the nuances of the clinical registry ecosystem and its diverse data sources to aid in the selection of the appropriate registry platform and to facilitate the seamless integration of modern technological solutions, thereby optimizing the flow of RWD into clinical research data. Such insight driven approaches will aid in gaining the full potential and value of RWD/RWE in supporting healthcare and research.
Disease foundations' clinical registry ecosystem
A foundation-funded strategic registry ecosystem includes three components: the foundation constituents and stakeholders component, the patients and advocacy group component, and the foundation registry hub component.
Source: Navitas Life Sciences
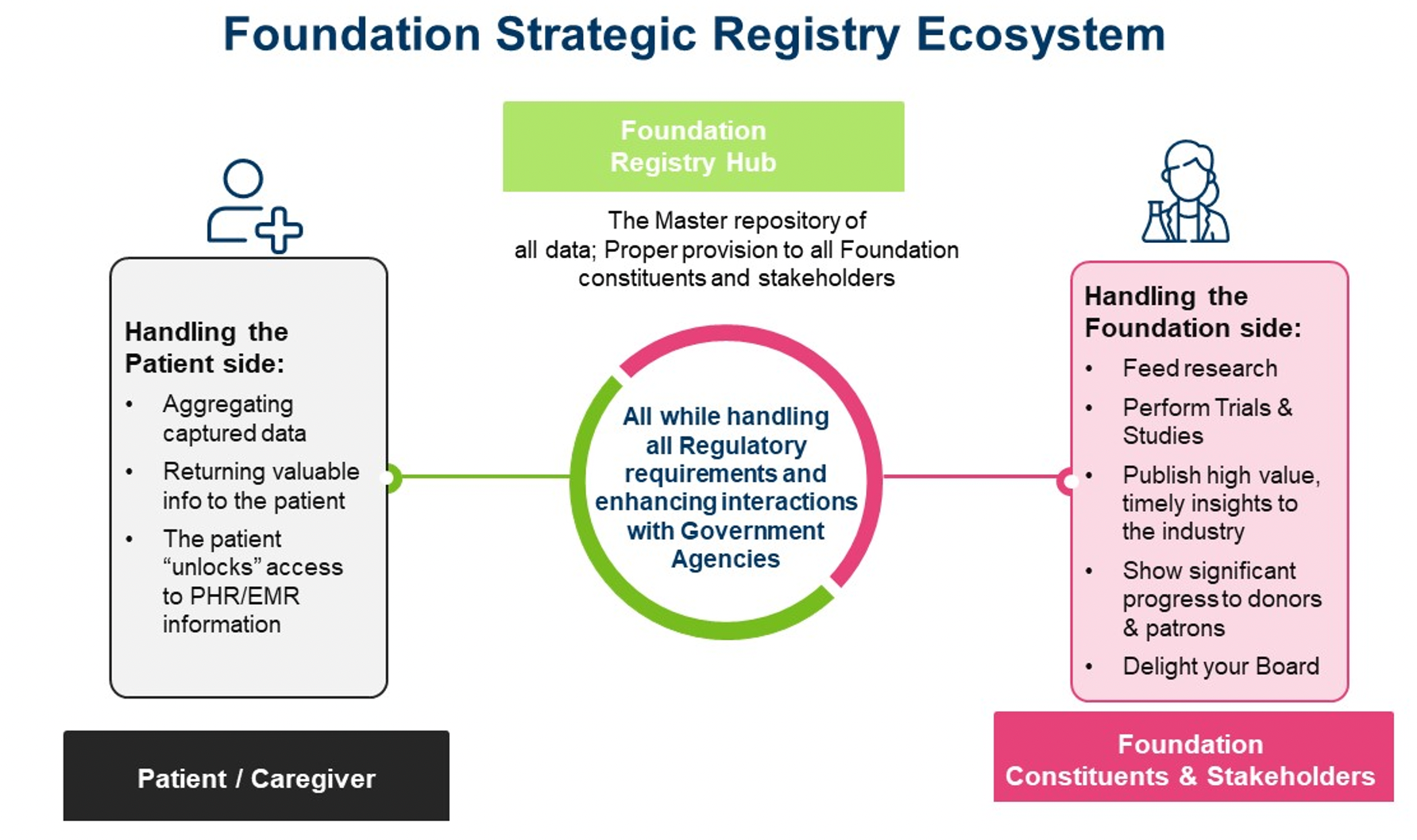
The foundation constituents and stakeholders component
A foundation is often under a lot of pressure when running a registry and/or any clinical research program due to:
- Financial challenges and stakeholder management: This includes funding limitations, and the need to satisfy patrons, donors, and endowments.
- Data management and processing requirements: There are often expectations for data aggregation and insights, therefore, the right eSolution choices, experience, and expertise in statistics and bioinformatics are needed to support data processing.
- Stakeholder accountability and partnership: Accountable to clinicians, scientists, and researchers, presenting incentives to your centers of excellence and/or sites, partnering with the government agencies and working with big pharmaceutical and biotech companies.
- Operational excellence and patient care: The need to run operationally excellent studies and programs, trying to find a cure and improved care for patients, and meeting/exceeding board expectations, are other imperatives.
Clinicians and researchers have strong motivations to participate in clinical registry programs, but can also encounter frustrations, including overwhelming amounts of information available to the registry study, the need for patient data to be re-entered in the registry systems, fragmented user experience lacking a streamlined workflow, and limited access to data insights. Operating a clinical registry across heterogeneous locations and clinical spaces can also present challenges for foundations due to inconsistencies in regulatory policies, clinical care practice, and rules and regulations regarding sharing of patient data. International clinical registries can be particularly complex in these areas.
The patients and advocacy group component
Patients, caregivers, and patient advocacy groups play an especially important role in the success of clinical registry programs.We will likely get patient data, but also need to make sure we protect patients’ privacy and return clinically relevant information back to them. To achieve long-term gains in improving health, disease management, and quality of life, it is imperative to keep patients engaged in their care and empower them with tools that support knowledge and education. Patient advocacy groups are a core component of many foundations, so it is important to consider their insights related to data obtained from clinical registries.
The foundation registry hub
The foundation registry hub includes the registry design and objectives, operation and regulatory support, valuable data returned to patients, patient education and clinical management, and support to ongoing and future clinical research and health care. The registry hub links and integrates foundation constituents and patient groups and makes sure a governance structure is in place to harmonize and integrate a registry program consistent with its purpose.
A highly skilled registry operation and data coordinating center (RODCC) usually offers the foundation and multiple stakeholder the most effective solution set. An RODCC’s deep experience in managing registry programs, building registry platforms, and supporting metadata driven data harmonization and system integration can be leveraged to develop and implement an outcome-based registry program. The solutions include:
- Efficient and effective full-service coordinating center support capabilities
- Operation and regulatory support, site support, monitoring system and system interoperability, data management, data harmonization and integration across multiple sources, data analysis and visualization
- Tailored capabilities catering to the client’s unique needs
Technology-enabled RWD and clinical research data integration
Clinical registry platform choices and components are very important to the success of the registry program. By consolidating data from a defined patient population based on RWE, the registry can help evaluate comparative effectiveness of treatment approaches, and guide clinicians toward targeted interventions for specific patient populations. RWD/RWE and clinical research data integration can help providers, health systems and payers, as well as patients.
The registry study plan and goals drive the selection of data source and platforms, influencing the decision-making process. Registries usually include data from electronic health record (EHR) systems. Additionally, data may also be collected directly from the patient in the form of patient reported outcomes, imaging data, genetic data, etc. Data standardization and common data elements (CDEs) become even more important to harmonize and integrate multiple data sources and meta data definitions. It is critical to work with your scientific leaders and subject matter experts (SMEs) to identify the important CDEs, data to be collected directly, address scientific queries, ensure accurate interpretation of the registry standard, and support data analysis and sharing.
The next step is to establish a clear understanding of the clinical registry data flow and necessary system features and capabilities, and to assess if these key features are commonly available in commercial products and/or if customization is needed.
Source: Navitas Life Sciences
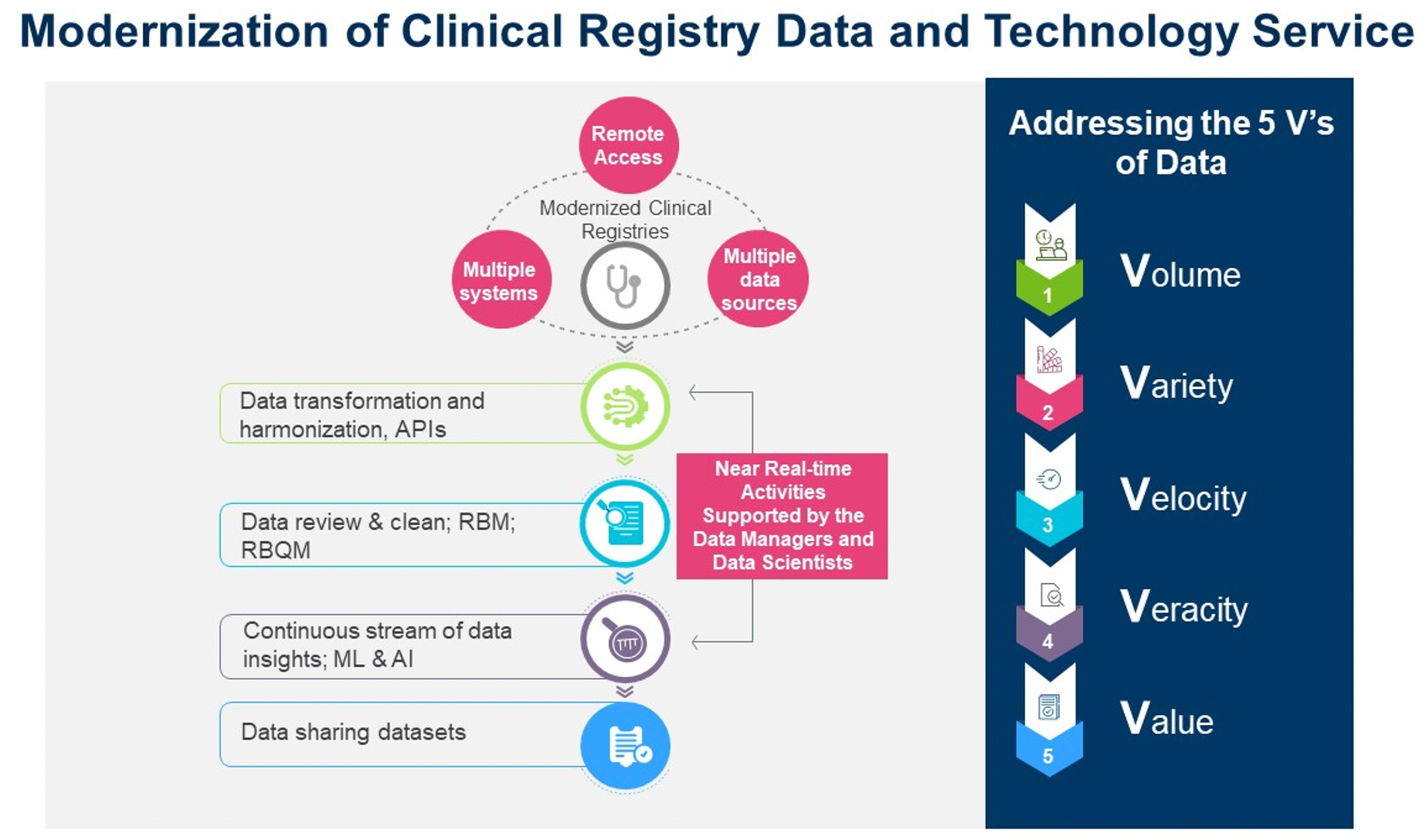
A scalable, configurable, and interoperable disease/patient registry platform can provide a dynamic, virtual environment where an enterprise can immediately access all of its data assets, plus all of its registries and analysis applications in one convenient location, facilitating health care and research collaboration and dissemination. A powerful eSolution platform needs to provide immediate access to all of its data assets, support metadata-driven data harmonization and integration, facilitate decentralized clinical trials (DCTs) and risk-based quality management (RBQM), data insights, and submission in one convenient location.
Modern clinical data flow and technologies must support the implementation of emerging data strategies and enable the aggregation, integration, and interpretation of high volumes of data, continually generated from many sources and multiple systems with complex data structures.
- EHR/electronic medical record (EMR) integration and harmonization: Carefully consider the EHR/EMR Integration and Harmonization approach. The health care and clinical data standards space continues to evolve rapidly. Research suggests that implementing therapeutic and disease specific CDEs and standards and re-using healthcare data for research can save study teams time and resources during start-up as well as during study conduct and analysis. API services and capabilities, i.e., Fast Healthcare Interoperability Resources (FHIR), can be used to support HL7 messaging and system interoperability.
- Foundation data warehouse: A registry data repository serves as the foundation data warehouse. This is why having a strategy of meta-data driven harmonized and standardized data elements is critical to support data exchange, interoperability, consistent interpretation and integration of data from disparate data resources. Furthermore, harmonized and consistent data can be seamlessly shared and is crucial to achieve analysis across different studies.
- Patient-specific registry dashboard: A registry dashboard is the representation of patient-specific data. This dashboard, along with broader data across all patients in the registry and information, from guideline and evidence sources, will then be efficiently delivered as clinical decision support through the clinician dashboard. Over time, this registry data content can also be made available outside the registry program. All the registry data can be made available in a de-identified manner to support translational research through extracts and reporting tools.
- Advanced statistical modeling and artificial intelligence (AI)/machine learning (ML) algorithms: Advanced statistical models and AI/ML algorithms are required to monitor data continuously throughout a trial as the complexity and volume of data expands.
- Flexibility for future data sources: The system needs to accommodate change since it is not possible to anticipate future data sources or elements that may be relevant to the continuous development of the registry. The stored data can be extracted and transferred to the statisticians, clinicians and researchers, patients, and others, as needed for analysis and data sharing.
- Specific module requirements: Special registry needs for specific modules, for example, eConsent, an imaging module, etc.
- Hosting, compliance, and support: Includes consideration of cloud hosting, HIPAA and other regulatory compliance, for example, 21CRF Part 11 and GDPR, etc.
- Cost and sustainability planning: Cost and sustainability is especially important to consider during the registry planning stage.
- Resources and skills for registry development: Last but not least, resources and specialties to develop a registry platform. Do you have internal resources and skills? If you have several registries, do you need a registry data coordinating center to support you to run an efficient and effective registry program?
Generative AI in healthcare and clinical research
Generative AI in healthcare and clinical research is expanding rapidly by generating content and results through analyzing large data and training examples. AI/ML libraries, automated process workflows, and notebook capabilities allow developers across the globe to work in tandem to author, revise, validate, and execute data models in real-time across a single study or program of studies. Digital platform delivers a streamlined and simplified solution for executing clinical registry study by using AI to integrate and automate processes from data aggregation to the generation of validation-ready datasets for data mining and insights.
Expanding adoption for clinical development
This modern clinical data flow and digital solution will support health care and research collaboration and dissemination, often serving as the study researcher portal. There are increased requests for the capability to support patients and caregivers' access to patients’ data through the patient dashboard, aiding in adaptive treatment and disease management. Many organizations and software vendors are placing increased effort into capturing clinical data from EHR systems across a wide array of products and settings. They have the experience in developing and successfully deploying registries and clinical decision support tools that are highly usable and embraced by clinicians and clinical researchers alike.
Harnessing RWD and utilizing technology provides high impact value. The drug development process will benefit significantly by building capabilities based on these insights, with immense value to patients.
Yun Lu, PhD, chief science and innovation officer, Susan Li, associate director, clinical data sciences, Sarah Lawrence, senior clinical project manager, and Sowmya Kaur, executive vice President; all with Navitas Life Sciences
Newsletter
Stay current in clinical research with Applied Clinical Trials, providing expert insights, regulatory updates, and practical strategies for successful clinical trial design and execution.
Managing Side Effects and Dosing in Off-Label GLP-1 Use with Help from Real-World Evidence
July 18th 2025Shipra Patel, global therapeutic area section head, endocrinology, global head, pediatrics, Parexel, explains how real-world data is helping researchers navigate gastrointestinal side effects, dose flexibility, and long-term tolerability in off-label GLP-1 use.
Unifying Industry to Better Understand GCP Guidance
May 7th 2025In this episode of the Applied Clinical Trials Podcast, David Nickerson, head of clinical quality management at EMD Serono; and Arlene Lee, director of product management, data quality & risk management solutions at Medidata, discuss the newest ICH E6(R3) GCP guidelines as well as how TransCelerate and ACRO have partnered to help stakeholders better acclimate to these guidelines.
Effect of AI/ML, Real World Evidence and Master Protocols on Trial Success
July 7th 2025How the application of artificial intelligence, broader use of real-world evidence, decentralized clinical trials, master protocols, and risk-based quality monitoring, together with strong ethical oversight and increased collaboration, are contributing to better healthcare delivery and strengthening the role of clinical research in driving global health progress.