
Assessing the benefits of using blockchain technology as a notary service in the network sharing of clinical data.

Assessing the benefits of using blockchain technology as a notary service in the network sharing of clinical data.

Adoption of the concept of Risk-Based Budgeting could help maintain the trial budget or even save the whole clinical trial.

The clinical trials industry craves blockchain technology to provide data safety and authenticity.
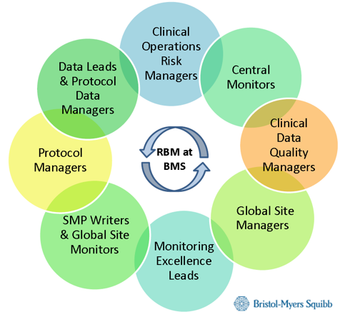
This article discusses the adoption of RBQM in academic settings and the lessons that were learned.

A look at the results of the recently completed project, which aimed to develop an holistic monitoring system via process integration to more effectively control clinical trial risks beyond isolated RBM strategies.
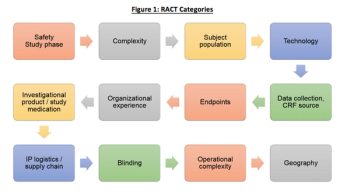
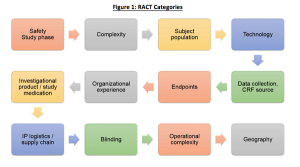
The biopharmaceutical industry is starting to adopt TransCelerate’s Risk Assessment Categorization Tool (RACT) in order to identify risks and plan a comprehensive clinical trial risk mitigation strategy. We recently wrote about the RACT moving to the cloud, and the advantages of using such systems. Some of these advantages include the ability for study teams to evaluate R&D portfolio risks by collecting and analyzing RACT data in aggregate.

In this article, you will find a structured summary and critical review of the new addendum, and as well as ideas on how to prepare for these regulatory changes.

Many of us speak about the importance of clinical trial data quality and integrity, yet the lack of data quality standards and definitions introduces subjectivity risk in clinical trials.
“Never assume that something obvious is true,”
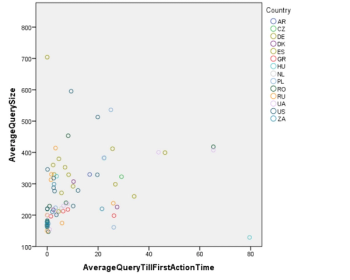
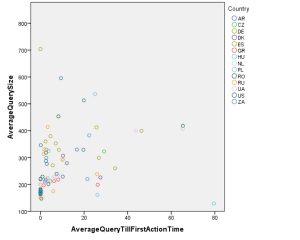
Clinical trial site engagement has been advocated as a critical component relating to a study’s performance and success, however, a minimum amount of data supports this connection.

This guidance document will offer information about RbM strategies.

Published: January 13th 2015 | Updated:
Published: March 19th 2015 | Updated:
Published: March 31st 2015 | Updated:

Published: May 19th 2015 | Updated:

Published: October 20th 2015 | Updated:
Published: May 11th 2016 | Updated: