Articles by Beat Widler, PhD

Endpoint adjudication is gaining importance in pharma today, however, no guidance on endpoint adjudication currently exists. The LinkedIn Endpoint Adjudication Community has generated an Events Charter Template to shed light on the subject for those in need of guidance.

TransCelerates work on their clinical trial Quality Management Systems (QMS) initiative began about one year ago. This article summarizes findings from a recent DIA publication about the initiative and evaluates potential issues during QMS framework implementation.
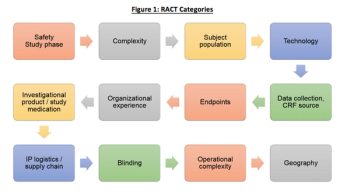
The biopharmaceutical industry is starting to adopt TransCelerate’s Risk Assessment Categorization Tool (RACT) in order to identify risks and plan a comprehensive clinical trial risk mitigation strategy. We recently wrote about the RACT moving to the cloud, and the advantages of using such systems. Some of these advantages include the ability for study teams to evaluate R&D portfolio risks by collecting and analyzing RACT data in aggregate.

In this article, you will find a structured summary and critical review of the new addendum, and as well as ideas on how to prepare for these regulatory changes.

The aforementioned article emphasized that a reduction in data quality ultimately leads to enrolling more clinical trial subjects in order to maintain equivalent statistical power.

RbM Guidance Document: Ten Burning Questions about Risk-Based Study Management
ByMoe Alsumidaie,Johanna Schenk, MD,Peter Schiemann, PhD,Beat Widler, PhD,Artem Andrianov, PhD,María Proupín-Pérez, PhD This guidance document will offer information about RbM strategies.