Articles by Peter Schiemann, PhD

TransCelerates work on their clinical trial Quality Management Systems (QMS) initiative began about one year ago. This article summarizes findings from a recent DIA publication about the initiative and evaluates potential issues during QMS framework implementation.
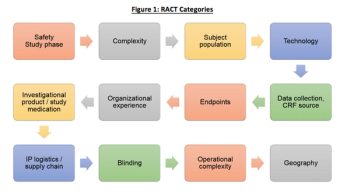
The biopharmaceutical industry is starting to adopt TransCelerate’s Risk Assessment Categorization Tool (RACT) in order to identify risks and plan a comprehensive clinical trial risk mitigation strategy. We recently wrote about the RACT moving to the cloud, and the advantages of using such systems. Some of these advantages include the ability for study teams to evaluate R&D portfolio risks by collecting and analyzing RACT data in aggregate.

An article recently appeared in Applied Clinical Trials, which indicated that oncology trials are taking longer to complete, and the article suggested that the duration of oncology clinical trials in certain phases increased by one year to one and a half years.1 Moreover, the article delineates that ameliorations in trial duration were most likely a result of increasing protocol complexi

The aforementioned article emphasized that a reduction in data quality ultimately leads to enrolling more clinical trial subjects in order to maintain equivalent statistical power.

RbM Guidance Document: Ten Burning Questions about Risk-Based Study Management
ByMoe Alsumidaie,Johanna Schenk, MD,Peter Schiemann, PhD,Beat Widler, PhD,Artem Andrianov, PhD,María Proupín-Pérez, PhD This guidance document will offer information about RbM strategies.