
Study Health Check provides early identification of issues related to protocol deviations and determines objective measures of site-specific versus study-wide performance.
MANA RBM, Denver, CO, USA

Study Health Check provides early identification of issues related to protocol deviations and determines objective measures of site-specific versus study-wide performance.

Identifying the ‘significant six’ areas of clinical study conduct to mitigate risk.
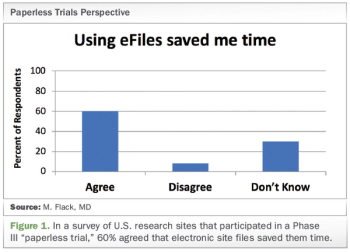
Exploring the use of electronic investigator site files for review of regulatory documents and informed consents.
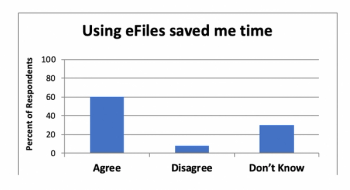
Penelope Manasco, CEO of MANA UBM, answer thorny questions about adopting electronic investigator site files in clinical research.

Penelope Manasco, CEO of MANA UBM, disputes recommendations made by the original authors she finds to be problematic and worthy of further discussion.

CEO of MANA UBM, Penelope K. Manasco, explores the different approaches to determine what the right 'easy button' is to push to achieve effective clinical trial conduct and oversight.

With the new regulatory guidelines concerning GCP, questions have arisen as to how best RBM plans can comply. These questions and answers will assist sponsors in assuring compliance.
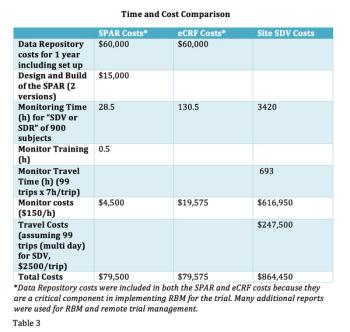
In a comparison study of source data review (SDV) methods, this new process demonstrates the value of using well-planned data visualization tools to provide better quality oversight versus remote eCRF review or onsite SDV.
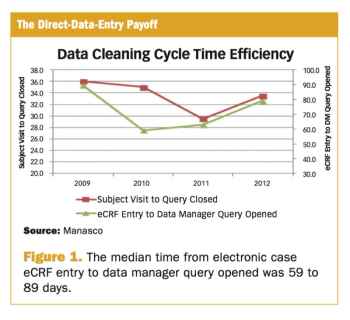
Outlining those technologies best able to raise the data and process quality of risk-based monitoring.

A risk-based approach to quality trial oversight, adopted by the FDA and EMA, has resulted in an array of new technology solutions being released.

Published: June 22nd 2023 | Updated:

Published: March 22nd 2021 | Updated:

Published: May 17th 2016 | Updated:
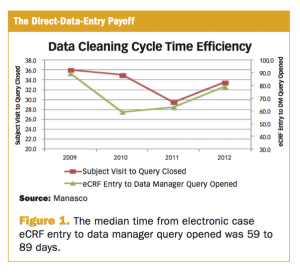
Published: June 1st 2016 | Updated:
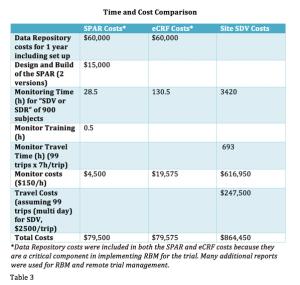
Published: December 2nd 2016 | Updated:

Published: January 30th 2017 | Updated: