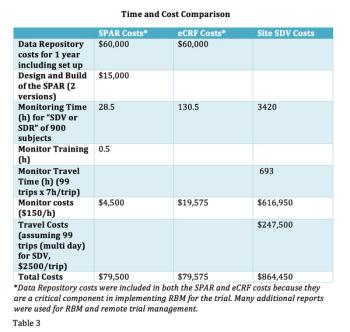
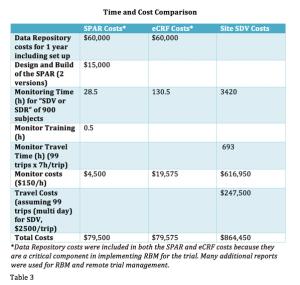
In a comparison study of source data review (SDV) methods, this new process demonstrates the value of using well-planned data visualization tools to provide better quality oversight versus remote eCRF review or onsite SDV.

In a comparison study of source data review (SDV) methods, this new process demonstrates the value of using well-planned data visualization tools to provide better quality oversight versus remote eCRF review or onsite SDV.
Published: December 2nd 2016 | Updated: