TTC
Articles by TTC

Tufts CSDD

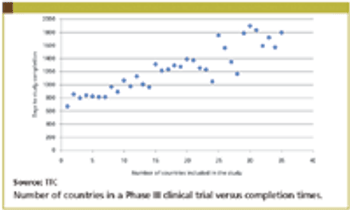
Improved study completion times represents one of the most critical areas for clinical development organizations. Previous TTC research has demonstrated that paying sites more on a cost per patient basis does not improve study completions times.
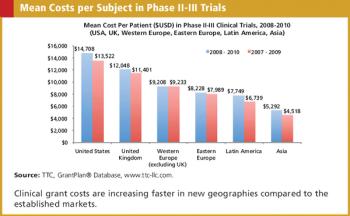
Clinical grant expenditures represent a major portion of the budget for later phase clinical trials.
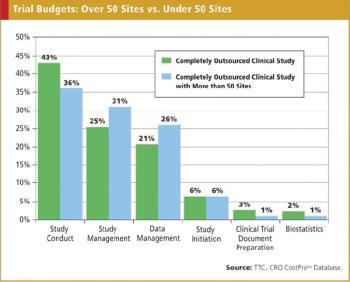
The use of CROs to conduct clinical trials, including data management, has increased over the years and will most likely continue to represent a significant part of many companies' clinical research efforts. Consequently, sponsor companies need to be able to budget the costs of these outsourced studies as effectively as possible. No one is pleased when a sponsor company requests bids for a study, or asks a preferred provider to submit cost estimates and the lowest submitted figures are substantially above the amounts expected by the sponsor company.
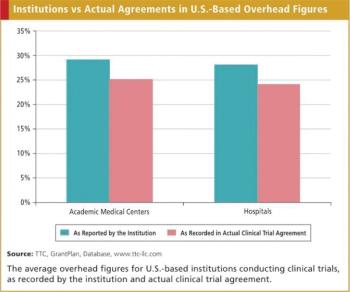
Perhaps few issues elicit more interest in clinical trial agreements (clinical grants) than the overhead portion of the contract. Clinical trial agreements typically have three parts: procedures, nonprocedures, and overhead.
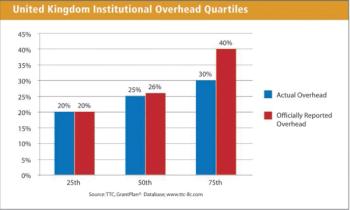
Institutional overhead remains one of the more contentious areas in clinical grant management. There can be substantial variations in these overhead rates from institution to institution with often times little immediately evident reason for the differences. Institution overhead rates in the United Kingdom seem to reflect that same imprecision.
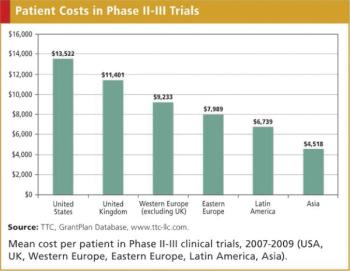
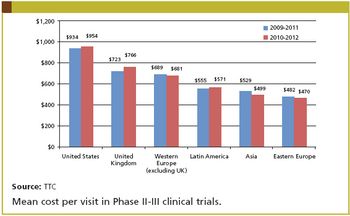
Clinical grant expenditures represent a major portion of the budget for later phase clinical trials. While the relative cost of conducting a clinical trial in a specific geography is not the sole driver in the decision of where to place study sites, relative costs do play a role.
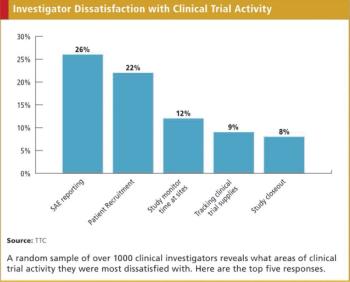
Drug safety has become a significant issue to many portions of society. High visibility issues like the Vioxx litigation have no doubt increased that salience among the general public. Moreover, with changing demographics, economic trends, and wider numbers of medical applications of prescription drugs, usage of these drugs will only increase, as will the accompanying issue of drug safety.
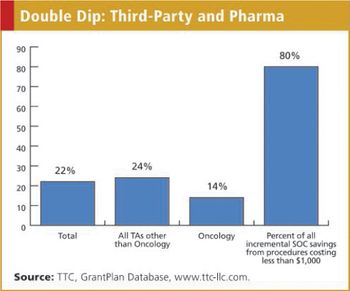
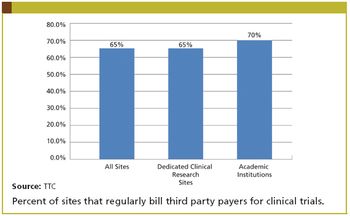
The financial aspect of standard of care in patient treatment has become a major issue for many companies conducting clinical research. Many industry monitors, self-appointed and otherwise, have highlighted the issue of payments to physicians by pharmaceutical companies, including the fair market payment of clinical grants, as possibly influencing physician behavior. Subjects in later phase clinical research must have the malady being treated in the clinical trial. Consequently, many of the treatments these patients receive would and should be covered by third-party payers, typically government agencies or commercial insurance companies.
Latest Updated Articles
 Revisiting Clinical Grant Expenditures
Revisiting Clinical Grant ExpendituresPublished: January 1st 2013 | Updated:
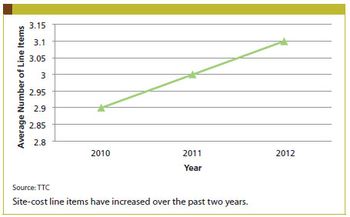
 Site-Cost Line Item Charges on the Rise
Site-Cost Line Item Charges on the RisePublished: October 1st 2012 | Updated:
 CNS Drugs Have a Low Approval Rate
CNS Drugs Have a Low Approval RatePublished: April 1st 2012 | Updated:
 Study Completion Times
Study Completion TimesPublished: May 1st 2011 | Updated:
 Standard of Care Billing More Widespread
Standard of Care Billing More WidespreadPublished: June 1st 2012 | Updated:
 Study Conduct: A Big Part of the Budget
Study Conduct: A Big Part of the BudgetPublished: May 1st 2010 | Updated:
