
Investigative Sites
Latest News
Latest Videos

More News

InterveXion CEO Keith Ward and Andrew Schafer, vice president of strategy for Biospatial discuss the details of a study which utilizes EMS data to identify sites for a Phase II clinical trial.

Industry-wide unity paves path for preparedness.

Bringing clinical trials to patients’ homes can address existing site-based challenges.

Lessons learned during the pandemic from University of Louisville.
While it's important to note that there are areas where the industry can improve, there are unique opportunities of improvement for specific types of sites and the number of studies conducted there.


Building authentic trust lays at the heart of creating more patient-centric trials.

Comparing late-stage COVID-19 vaccine trials to historical practices.
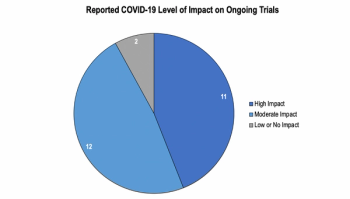
Findings from a Tufts study examining the effects of COVID-19 on clinical trials.
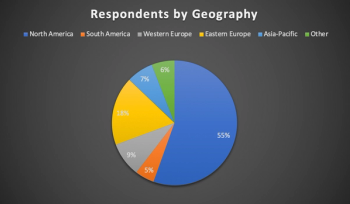
Lessons from six months of conducting clinical trials during the COVID-19 pandemic

Hassan Kadhim, Director, Global Head of Clinical Trial Business Capabilities, Clinical Innovation & Industry Collaborations at Bristol Myers Squibb, discusses his perspectives on how the pandemic has shifted clinical innovation.

Recognizing pre-pandemic pain points, such as patient engagement and protocol development, could lead to post-pandemic trial success.

Exploring effective strategies for sponsors and CROs to ensure both their CRAs and sites are supported for high levels of site acceptance and streamlined remote monitoring.

The pandemic has thus far disproportionally impacted minority populations, and our ongoing failure to adequately represent all patients regardless of demographic background has never been more important to remedy than it is today, writes ACRP Workforce Innovation Officer Beth Harper.

Creative approaches are needed to address the clinical research workforce “talent wars”

Exploring how clinical research sites are redefining their business models to be more flexible, collaborative, and customized.

A case study of the Parkinson’s Progression Markers Initiative.
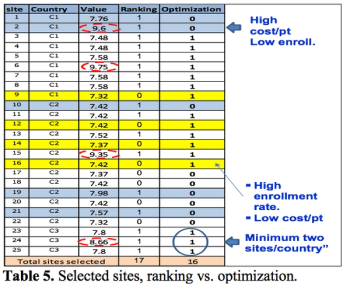
Site selection is one of the most important and at the same time challenging problems in clinical trials planning. Poor site selection may cause enrollment delays, resource waste on low or zero enrollment, and even potentially compromise trial results.

In this article, Moe Alsumidaie will discuss how my study teams manage monitoring reports and offer a tracking tool to assist sites with the process.

Examining the practicality of implementing CM techniques to drive trial oversight efficiency while saving on-site monitoring resources and costs.

The last time the ICH GCP Guideline was updated, the process of conducting a clinical trial, including risk assessment and monitoring, was a largely paper-based affair. Since then clinical trials have evolved substantially as they’ve become more complex, costly, and global in scope.

In this interview, Christa Polidori, Clinical Trial Disclosure Manager at Bristol-Myers Squibb and a leader for the TransCelerate Clinical Research Access and Information Exchange Initiative, will discuss the TransCelerate proposal in greater detail.
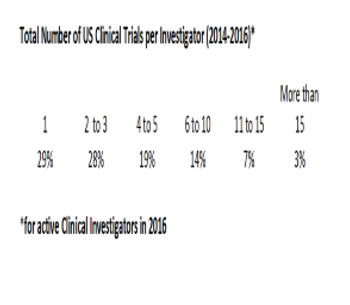
This data analysis takes a look at how much experience US clinical investigators have.

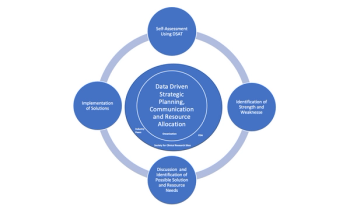
A model to identify the services and resources sites need to conduct high-quality clinical trials.
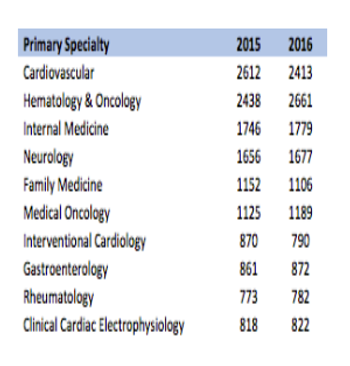
Estimates of active US investigators range from about 20,000 to almost 150,000.












