
A look into the evolving relationship and what motivates the two to work together.

A look into the evolving relationship and what motivates the two to work together.

Concerns are being put into action with the trend toward better investigator training.

Ways to ensure the transition between vendors is as smooth as possible for everyone involved.

It doesn't have to be, but they do require an integrated approach that stresses quality assurance.

A look into how flow cytometrycan benefit global clinical trials and what an IT savvy central lab can contribute.

Mobile phones enable innovative tele-, home-, and health-monitoring solutions for clinical trial subjects.

How this electronic data capture technology enhanced clinical trial design for two allergy studies.

Integrating a study's eClinical systems enables more effective management and eliminates pesky redundancies.

The potential for transformation within the clinical research process is here and now. Are you ready?
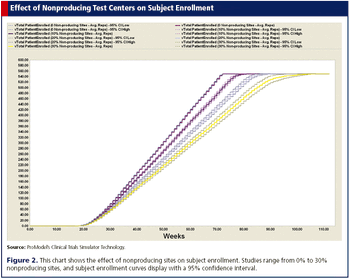
The use of computer simulation models to improve both site selection and subject recruitment.
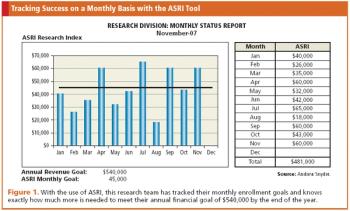
Real-time financial performance indicator for clinical research sites that answers the question, how are we doing?

The impact of proactive subject recruitment planning.
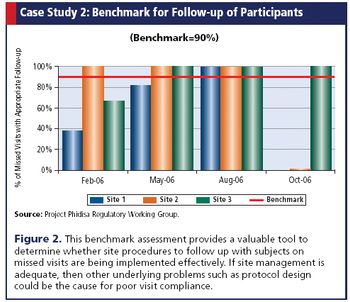
How one research project in South Africa developed its own benchmark assessment tool to gauge performance and compliance across sites.

Today's problem-plagued feasibility assessment process is in need of repair.
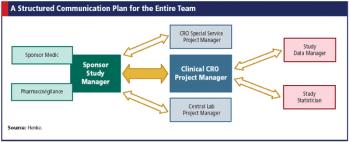
Just like pharma, biotech companies need clinical research organizations too, but are their needs different?

Why this age-old industry staple is missing the mark for many and what can be done to remedy the situation.
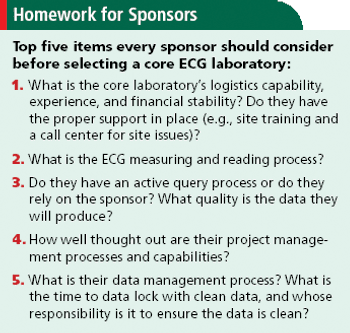
A call to sponsors to rethink the role of ECGs in drug development and the use of central core labs.

Pilot test to rebrand clinical research shows promise as a way to build public trust and promote interest.
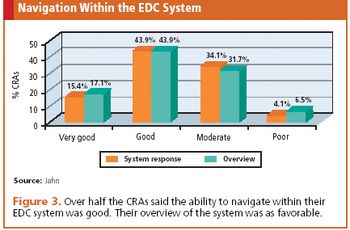
According to results from a recent survey, clinical research is poised for the eClinical journey ahead.

The decision to outsource must also consider not so obvious issues that are as critical as cost.

Today's CROs need to provide expertise on a global scale.
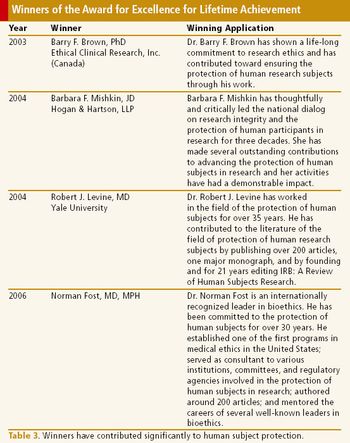
Award for excellence in human research protection is the incentive for better oversight and regulations.

How text messaging could revolutionize clinical trial subject recruitment and compliance.
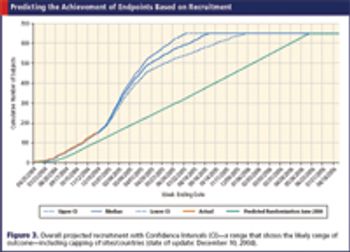
In the age of international trials, data drives the selection of golden sites and investigators to get it right.

Carl Anderson, senior consultant for Biologics Consulting Group, addresses the frequent overuse of Notes to File and concludes that documenting a mistake means absolutely nothing during an FDA inspection.