
Carl Anderson
Advertisement
Articles by Carl Anderson

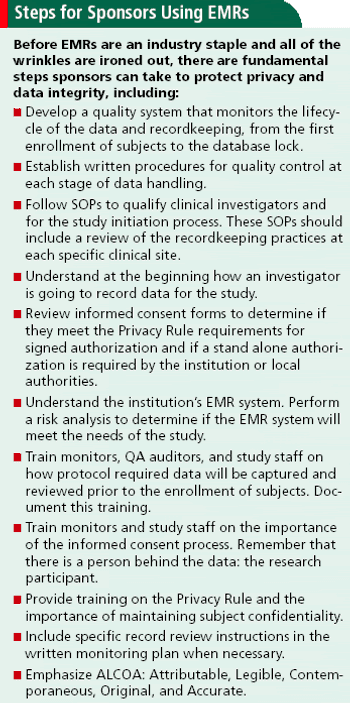
Shedding light on common EMR concerns about privacy, government regulations, and data integrity.

Carl Anderson, senior consultant for Biologics Consulting Group, addresses the frequent overuse of Notes to File and concludes that documenting a mistake means absolutely nothing during an FDA inspection.
Advertisement
Latest Updated Articles
 Key Updates to the 1572
Key Updates to the 1572Published: May 23rd 2012 | Updated:
 Note to File (NTF) is Not an FDA Panacea
Note to File (NTF) is Not an FDA PanaceaPublished: March 1st 2008 | Updated:
 The Ins and Outs of Electronic Medical Records
The Ins and Outs of Electronic Medical RecordsPublished: September 1st 2008 | Updated:
Advertisement
Advertisement
Trending on Applied Clinical Trials Online
1
SCOPE Summit 2026 Keynote Panel: Is Radical Acceleration in Clinical Research Possible?
2
SCOPE Summit 2026 Panel Discussion: Diversity in Clinical Trials—What’s Working, What’s Next
3
SCOPE Summit 2026: Reducing Patient Burden Is the Foundation of Wearable Success in Oncology
4
Accelerate Clinical Trials with AI-Enhanced Financial Management
5
