
Industry must grasp the cultural nuances of these two burgeoning countries to find success there.

Industry must grasp the cultural nuances of these two burgeoning countries to find success there.

Matt Kibby, leader of global operations for BBK Worldwide, discusses why country study managers hold the key to global enrollment success but are afraid to use it.

How imaging CROs minimize data variability in multisite studies and create the necessary consistency.

Perceptive Informatics Improves the Clinical Development Process with Enhanced CTMS Technology
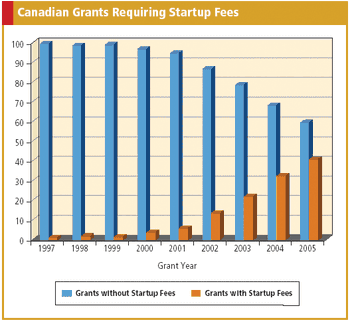
Data confirms more sites are asking for separate startup fees and that oncology trials are leading the way.
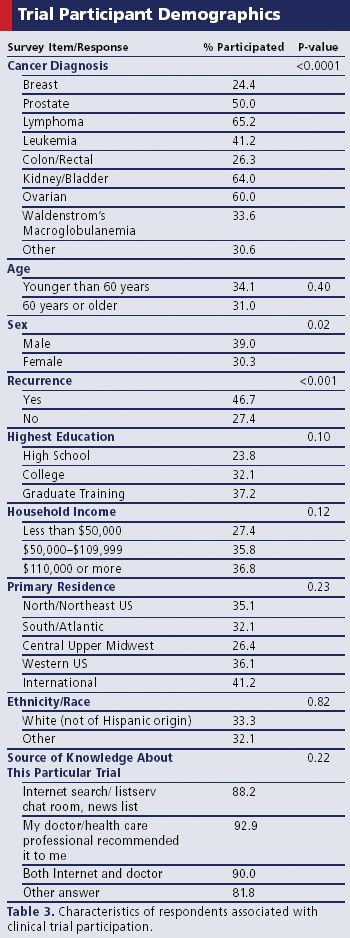
To improve enrollment in oncology trials, one patient advocate group went straight to the source.

Today's CRAs must redefine their roles in the face of changing industry expectations and new technologies.

How a good plan and hot lunch can spell success for sponsors. Attention to detail can help boost dwindling subject enrollment and retention, saving both time and money.

In addition to study timelines and budgets, EDC adoption directly affects the people who run trials.

India's investigators play a key role in helping CROs & sponsors recruit patients and meet global standards.

A new patient recruitment campaign depicts trial volunteers as heroes and aims to both educate and win over the public.
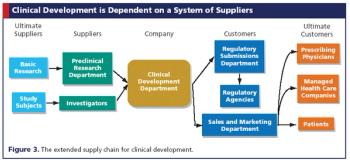
Applying supply chain management principles to clinical development can save sponsors money and time.
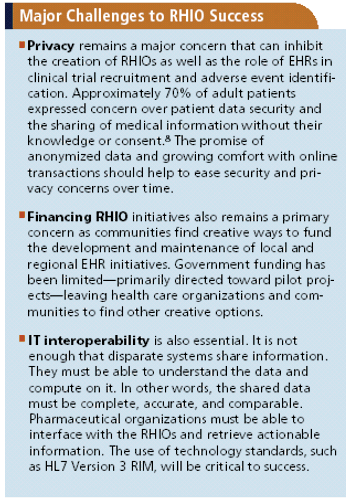
EHR has the potential to help solve long-time clinical trial recruitment, cost, and safety challenges.

India continues to gain the attention of pharmaceutical companies looking for a place to conduct clinical trials because of the country's reputation for fast subject recruitment rates. But as Jane Barrett reveals, sometimes the country's clinical trial participants are more uninformed than informed.

The importance of human subject protection, clear communication, and community spirit are among the issues emerging from the tragedy.

Yesterday's SMOs have given way to a new service provider that should be recognized.
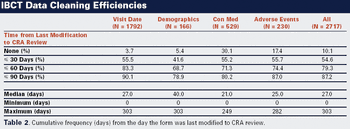
This case study shows how Internet-based clinical trials improve data entry, monitoring, and management.

Teaming up with a staffing partner can help companies find the right CRA for their trial.
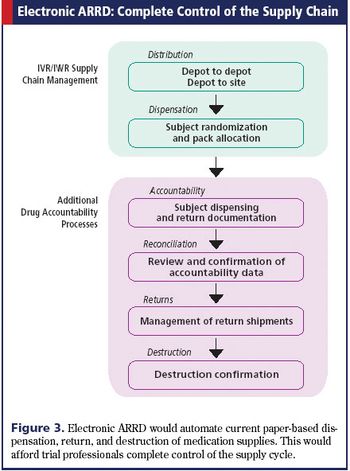
Electronic solutions can enhance the efficiency of tracking drugs throughout a clinical trial.

Common barriers to enrollment can be overcome by using targeted recruitment strategies.

Electronic health records are a viable alternative to today's subject recruitment methods.

Study findings show class attendance improves the outcome of CRA audits.

The single most important need for integrating EHR and EDC is the elimination of redundant data entry.

A common set of rules for ensuring a two-way flow of information and addressing subjects' needs.

With the help of Microsoft, Broadpeak and DataLabs prove seamless integration is possible.