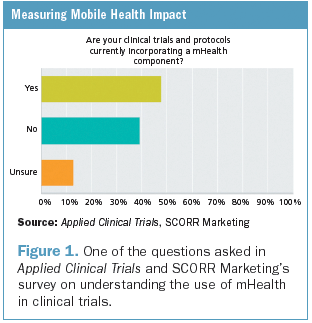
Investigative Sites
Latest News
Latest Videos
More News

Asia Pacific and US physicians' attitudes toward involvement in clinical trials.

Prior to initiating a trial with ED sites, three factors must be considered in order to achieve success.

Sponsors, CROs, and investigator sites must all work together for effective patient recruitment.
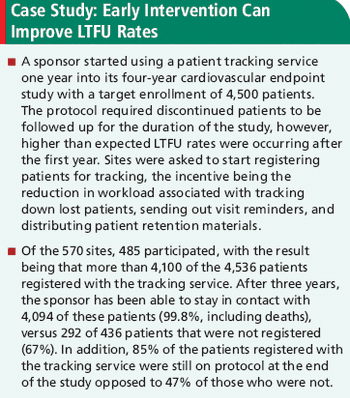
A 10-fold reduction in LTFU rates was observed in clinical trials that used patient tracking services.

Research sponsors and the research community must solve the inefficiency and redundancy that plagues the ethics review system.

Why are some contract research organizations, sponsors, and other research facilities not pursuing accreditation?

Navigating state and local laws and protecting the rights of human subjects are two of the many benefits of independent IRBs.
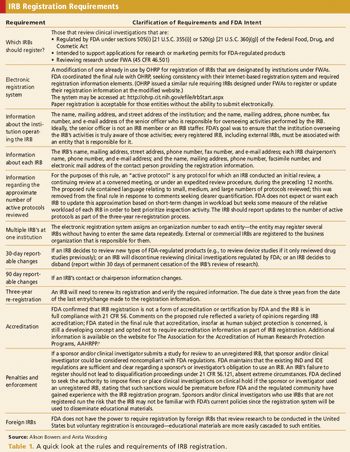
Registration is required for FDA-regulated IND studies, but is it needed for non-IND studies?

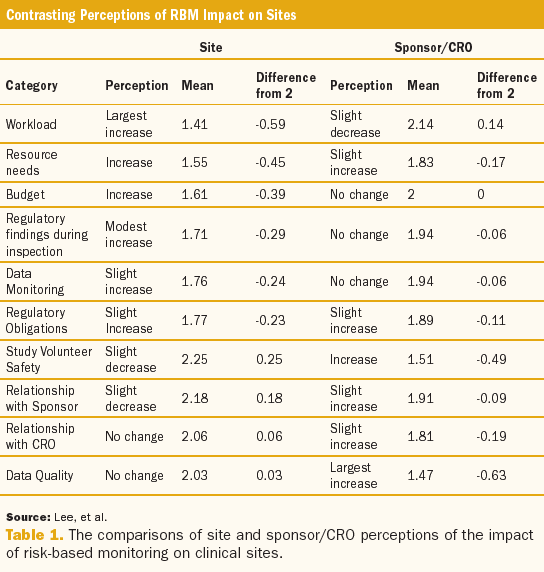
Monitoring of clinical trials is a federally mandated responsibility of trial sponsors and a core offering of contract research organizations (CROs) that is crucial to the validity of clinical research.

Recession and acquisitions make recruiting America's second-largest minority even harder.

It is necessary to understand the time, staff, and financial resources required to conduct clinical trials.
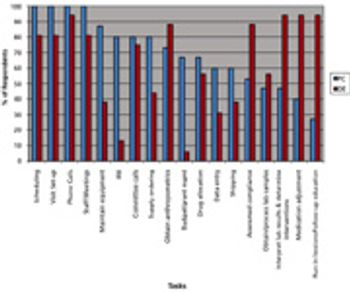
Reducing the challenges of study coordination in multi-site clinical trials.

Survey reveals industry practices surrounding standard of care and insurance claims data.

Clinical trial management systems seamlessly support enhanced financial productivity for sites.

Integration of EHR-EDC data could chang the way clinical research approaches subject recruitment

Patients share factors that most inform, educate, and motivate clinical trial participants.

A look at the past, present, and future of interoperability across the clinical research spectrum.

As people flock to the Internet for health information, the Web grows as a patient recruitment pathway.

Strategies to successfully manage the business of clinical trials in today's environment.

Detailed instructions for creating strong SOPs that can serve as the backbone of studies and sites.

Proposed "lottery" or deferred payment model aims to resolve undue inducement.

European community weighs in on challenges to pediatric research in this survey from EUCROF.

Advancing medicine and the clinical research process with the help of patient organizations

Overcoming hurdles in subject enrollment by managing potential risks in six critical key areas.

A look into the evolving relationship and what motivates the two to work together.