
Consortium looks to advance endpoint assessment through the development of eCOA best practices.

Consortium looks to advance endpoint assessment through the development of eCOA best practices.

Understanding differences in studies, sites, and patients are key to making eConsent successful.

Breaking down the barriers to eConsent adoption.

Finalizing protocols, aligning teams, and staying engaged headline best practices.

Six best practices and three key mistakes to avoid in new technology space.

Phase I/II study in cancer patients showcases eClinical effectiveness.

New requirements must be put in place to ensure data quality and integrity.

How site management team leader Emma Earl, a 2021 Veeva R&D Hero, is developing a culture of improvement at Bayer, or what Japanese business strategists have long called Kaizen, in its clinical operations.
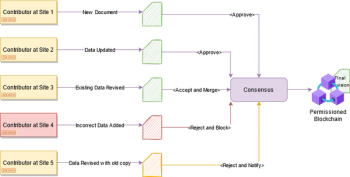
eTMF-blockchain technology offers a myriad of application benefits to data quality and integrity while ensuring compliance to ethical standards.
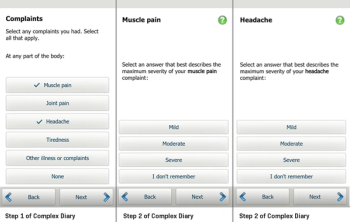
Sponsors can simplify diaries by taking a closer look at the three common sources of complexity.

Guidance on the unique challenges presented by electronic outcomes.

Elizabeth Rickenbacher, PhD, Director of Strategy for 4G Clinical discusses changes in the IRT and RTSM solutions.

Denis Polyanskiy, founder of Trialcome, discusses how the industry can overcome issues with non-adherence.

Jeff Wiley, head of oncology, global clinical project delivery at Labcorp Drug Development, sheds light on how Labcorp Drug Development is using remote monitoring to advance the industry toward new levels of connectivity.
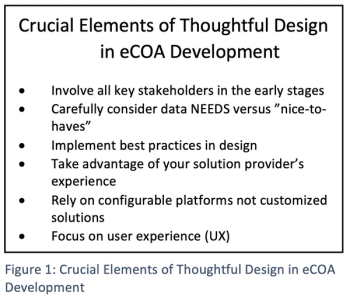
To derive the greatest benefits from eCOA technology, several elements must work together to create a strong foundation for sponsor success.
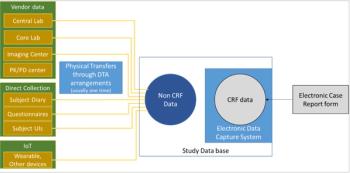
While Case Report Forms are a main contributor to collected data, non-CRF data such as core laboratory data and central imaging can be critical to any clinical study.

Lessons learned during the pandemic from University of Louisville.

A discussion of the regulatory history behind audit trails and its interpretation as it relates to clinical research data originating or residing in EDC or eCOA systems.

Charles Sydnor, CCRA, ACRP-CP, project manager at Crofoot, discusses how offering a digital patient experience in trials enables the site to provide better patient care.

There's no one-size-fits-all approach during the COVID-19 pandemic

To derive the greatest benefits from eCOA technology, several elements must work together to create a strong foundation for sponsor success.

New technological solutions for both patients and HCPs create a well-rounded eClinical approach

eClinical, eSource and EHRs is the journey to the holy grail of data efficiency in clinical trials.

Shiri Diskin, PhD, Head of Medical Writing at Bioforum, sits down with Daphna Laifenfeld, PhD, Chief Scientific Officer at Ibex Medical Analytics to discuss the challenges associated with developing a CER and how to effectively navigate them while creating opportunities that increase efficiencies.

With an increasing amount of diverse data that must now be collected and analyzed, the industry is faced with increasingly complex studies that present new challenges in data management.