
Examining how to optimizing the time and effort of Ethics Committees to efficiently and effectively fulfill their human subjects protection remit.

Examining how to optimizing the time and effort of Ethics Committees to efficiently and effectively fulfill their human subjects protection remit.
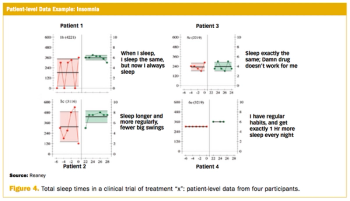
Measuring clinically relevant response requires routine and regular collection of outcomes data.

The authors contrast two views on the regulations concerning electronic source data.

Published: August 14th 2020 | Updated:

Published: June 1st 2002 | Updated:
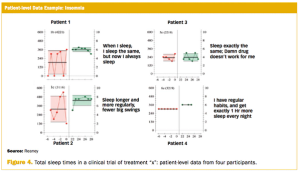
Published: February 1st 2016 | Updated: