
EDC technology is only one component in the transition to a viable electronic existence.

EDC technology is only one component in the transition to a viable electronic existence.

To capitalize on technology, a new approach to managing data is needed. Peg Regan, chief executive officer for PharmaPros Corporation, explains the concept of Electronic Data Lifecycle Management and how it will improve the quality of data and the time it takes to access it.

Fresh and flashy may appeal to the senses, but when it comes to trial software, it's experience that counts.

Today's technology narrows the gap between Web and desktop applications, transforming the user experience.
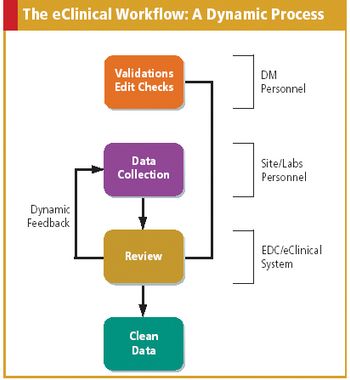
As more companies adopt eClinical technologies, data managers must redefine and update their role.

From blogs to social networks, the newest Web is redefining the way we use technology.

Software as a service might be the future of computing, but it won't reside on desktops.

Leveraging technology is key to fulfilling the promise of automation and streamlining clinical trials.
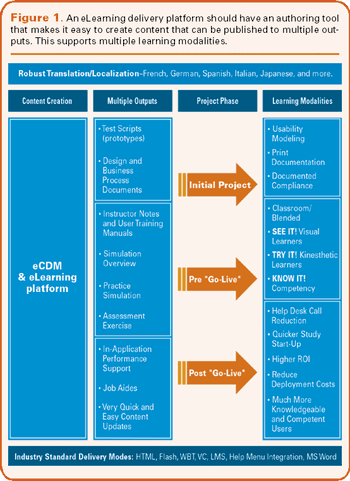
One of the most powerful features of a good eLearning solution is the ability for users to repeat a training course and take a test repeatedly to gain mastery of the material.

A story about 1/2-inch holes and the power of liquid soap

This new model standardizes data exchange between the various stages of a research study.

Making important data more consistent and more accessible has the potential to improve safety and efficacy.

The FDA Initiative represents the challenge facing our industry.

The clinical trial industry can thrive by offering easy-to-use technology to make data available earlier.

Writing computer code isn?t as difficult as you might think.
The benefits offered by the iCDW begin with a single trial by accelerating the statistical analysis plan.
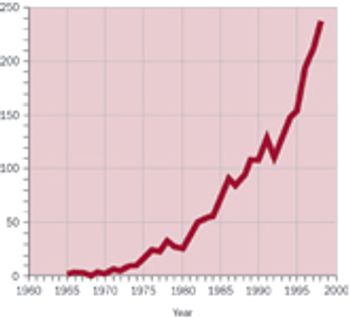
Estimates suggest that biopharmaceutical companies spend more than $150 million per year on data exchange. The industry needs to work smarter, faster, and with fewer resources.

A technological look at yesterday and today could predict tomorrow's trends.

Paper-free clinical trials may not ever happen-but many of its advantages are already available.

For all the promises of the impact of technology on clinical trials-from enhanced trial efficiencies to reduced time to market-very few specific examples have been published that clearly demonstrate a marked impact. This article provides just such an example, by examining the impact of electronic diaries (eDiaries) on the collection of Patient Reported Outcome (PRO) data in a large, pivotal Phase III trial evaluating a medication for the treatment of overactive bladder (OAB). We compare the results obtained using eDiaries to capture real-time data on voluntary and involuntary micturitions with those obtained from a Phase II trial that used paper diaries. The result: measurable subject protocol compliance and significantly reduced error variance, yielding increased study sensitivity and the opportunity to realize a significant financial savings in future trials.

One new idea that?s ready for use; another that will be soon; and a third that?s not yet there.

Two of the latest concepts in computing are new twists on old ideas?which could be the eventual path for clinical trials data processing.

Web services and the concept of technology integration are hot new trends that may have a major impact on the way we do business and manage data.

Paul Bleicher shares his regulatory philosophy and perspective and defends his position regarding eSource, in response to June?s Guest Commentary.

Take a tour through the world of freeware, shareware, shrink-wrapped, enterprise, and open source software.